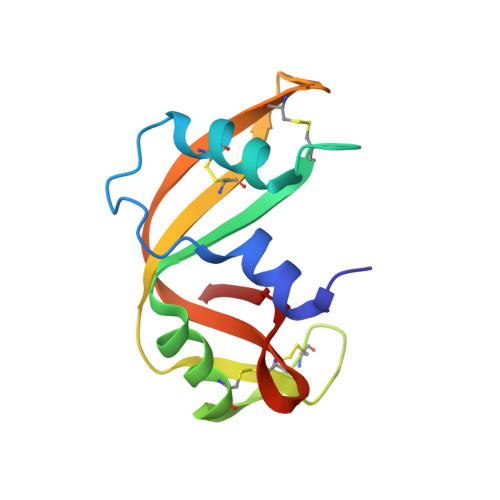
The crystal structure of recombinant rat pancreatic RNase A.
Gupta, V., Muyldermans, S., Wyns, L., Salunke, D.M.(1999) Proteins 35: 1-12
- PubMed: 10090281
- DOI: https://doi.org/10.1002/(sici)1097-0134(19990401)35:1<1::aid-prot1>3.0.co;2-2
- Primary Citation of Related Structures:
1RRA - PubMed Abstract:
The three-dimensional structure of rat pancreatic RNase A expressed in Escherichia coli was determined. The backbone conformations of certain critical loops are significantly different in this enzyme compared to its bovine counterpart. However, the core structure of rat RNase A is similar to that of the other members of the pancreatic ribonuclease family. The structural variations within a loop bordering the active site can be correlated with the subtle differences in the enzymatic activities of bovine and rat ribonucleases for different substrates. The most significant difference in the backbone conformation was observed in the loop 15-25. This loop incorporates the subtilisin cleavage site which is responsible for RNase A to RNase S conversion in the bovine enzyme. The rat enzyme does not get cleaved under identical conditions. Molecular docking of this region of the rat enzyme in the active site of subtilisin shows steric incompatibility, although the bovine pancreatic ribonuclease A appropriately fits into this active site. It is therefore inferred that the local conformation of the substrate governs the specificity of subtilisin.
Organizational Affiliation:
Structural Biology Unit, National Institute of Immunology, New Delhi, India.