Crystal structure of a novel antifungal protein distinct with five disulfide bridges from Eucommia ulmoides Oliver at an atomic resolution.
Xiang, Y., Huang, R.H., Liu, X.Z., Zhang, Y., Wang, D.C.(2004) J Struct Biol 148: 86-97
- PubMed: 15363789
- DOI: https://doi.org/10.1016/j.jsb.2004.04.002
- Primary Citation of Related Structures:
1P9G - PubMed Abstract:
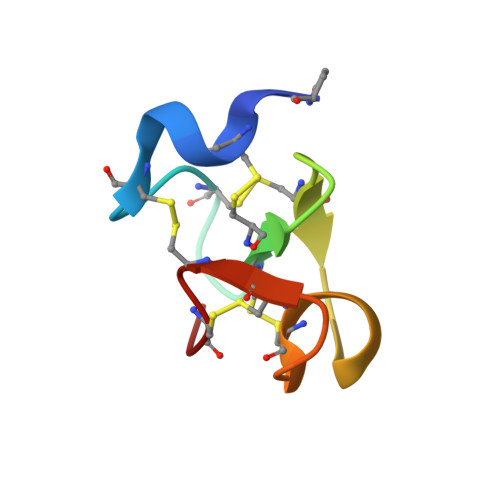
EAFP2 is a novel antifungal protein isolated from the bark of the tree Eucommia ulmoides Oliver. It consists of 41 residues and is characterized with a five-disulfide motif and the inhibitory effects on the growth of both cell wall chitin-containing and chitin-free fungi. The crystal structure of EAFP2 at an atomic resolution of 0.84 A has been determined by using Shake-and-Bake direct methods with the program SnB. The phases obtained were of sufficient quality to permit the initial model built automatically and the structural refinement carried out using anisotropic displacement parameters resulted in a final crystallographic R factor of 6.8%. In the resulting structural model, all non-hydrogen protein atoms including an unusual pyroglutamyl acid residue at the N-terminal can fit to the articulated electron densities with one centre and more than 65% of the hydrogen atoms in the protein can be observed as individual peaks in the difference map. The general fold of EAFP2 is composed of a 3(10) helix (Cys3-Arg6), an alpha-helix (Ala27-Cys31) and a three-stranded antiparallel beta-sheet (Cys16-Ser18, Cys23-Ser25, and Cys35-Cys37) and cross-linked by five disulfide bridges. The tertiary structure of EAFP2 can be divided into two structural sectors, A and B. Sector A composed of residues 11-30 adopts a conformation similar to the chitin-binding domain in the hevein-like proteins and features a hydrophobic surface embraced a chitin-binding site (Tyr20, 22, 29, and Ser18). The distinct disulfide bridge Cys7-Cys37 connects the N-terminal ten residues with the C-terminal segment 35-41 to form the sector B, which features a cationic surface distributing all four positively charged residues, Arg6, 9, 36, and 40. Based on these structural features, the possible structural basis of the functional properties of EAFP2 is discussed.
Organizational Affiliation:
Center for Structural and Molecular Biology, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, People's Republic of China.