Crystallographic structure of a PLP-dependent ornithine decarboxylase from Lactobacillus 30a to 3.0 A resolution.
Momany, C., Ernst, S., Ghosh, R., Chang, N.L., Hackert, M.L.(1995) J Mol Biol 252: 643-655
- PubMed: 7563080
- DOI: https://doi.org/10.1006/jmbi.1995.0526
- Primary Citation of Related Structures:
1ORD - PubMed Abstract:
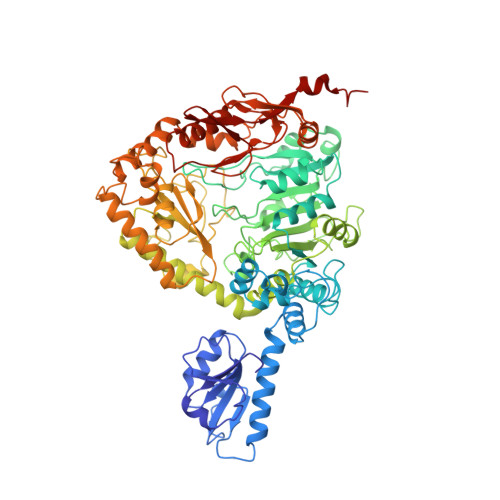
Ornithine decarboxylase from Lactobacillus 30a (L30a OrnDC) is representative of the large, pyridoxal-5'-phosphate-dependent decarboxylases that act on lysine, arginine or ornithine. The crystal structure of the L30a OrnDC has been solved to 3.0 A resolution using MIR phases in combination with density modification (space group P6; a = 195.6 A, c = 97.6 A; dimer of 1460 amino acid residues/asymmetric unit; VM = 3.26 A3/Da). The refined crystallographic R-value was 0.219 (Rfree = 0.268) using 2-fold restraints with a 4 sigma cutoff and 8.0 to 3.0 A resolution data. Six dimers related by C6 symmetry compose the enzymatically active dodecamer (approximately 10(6) Da). Each monomer of L30a OrnDC can be described in terms of five sequential folding domains. The amino-terminal domain, residues 1 to 107, consists of a five-stranded beta-sheet termed the "wing" domain. Two wing domains of each dimer project inward towards the center of the dodecamer and contribute to dodecamer stabilization. The "linker" domain, residues 108 to 160, consists of short alpha-helices separated by a loop that fills in the PLP pocket. The third domain, residues 161 to 413, is an alpha/beta domain containing a seven stranded beta-sheet that resembles the PLP-binding domain of the aspartate aminotransferases. The fourth domain, residues 414 to 569, resembles the "small" domain of the aspartate aminotransferases, but is significantly larger due to insertions. The remaining carboxy-terminal domain, residues 570 to 730, is organized into multiple antiparallel loops and seven alpha-helices that help form a deep channel leading to the PLP-binding site.
Organizational Affiliation:
Department of Chemistry and Biochemistry, University of Texas at Austin 78712, USA.