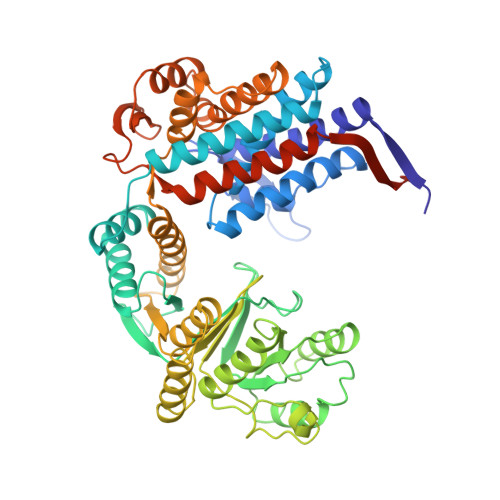
Domain Motions in GroEL upon Binding of an Oligopeptide.
Wang, J., Chen, L.(2003) J Mol Biol 334: 489-499
- PubMed: 14623189
- DOI: https://doi.org/10.1016/j.jmb.2003.09.074
- Primary Citation of Related Structures:
1MNF - PubMed Abstract:
GroEL assists protein folding by preventing the interaction of partially folded molecules with other non-native proteins. It binds them, sequesters them, and then releases them so that they can fold in an ATP-driven cycle. Previous studies have also shown that protein substrates, GroES, and oligopeptides bind to partially overlapped sites on the apical domain surfaces of GroEL. In this study, we have determined the crystal structure at 3.0A resolution of a symmetric (GroEL-peptide)(14) complex. The binding of each of these small 12 amino acid residue peptides to GroEL involves interactions between three adjacent apical domains of GroEL. Each peptide interacts primarily with a single GroEL subunit. Residues R231 and R268 from adjacent subunits isolate each substrate-binding pocket, and prevent bound substrates from sliding into adjacent binding pockets. As a consequence of peptide binding, domains rotate and inter-domain interactions are greatly enhanced. The direction of rotation of the apical domain of each GroEL subunit is opposite to that of its intermediate domain. Viewed from outside, the apical domains rotate clockwise within one GroEL ring, while the ATP-induced apical domain rotation is counter-clockwise.
Organizational Affiliation:
Department of Molecular Biophysics and Biochemistry, Yale University, 266 Whitney Avenue, Bass Center, Rm 418, New Haven, CT 06520-8114, USA. wang@mail.yale.edu