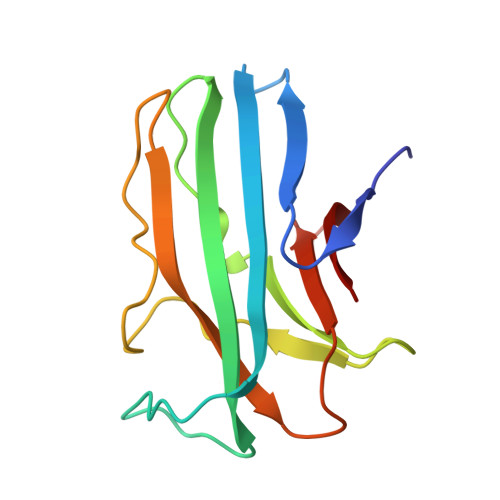
Crystal structure of a major secreted protein of Mycobacterium tuberculosis-MPT63 at 1.5-A resolution
Goulding, C.W., Parseghian, A., Sawaya, M.R., Cascio, D., Apostol, M., Gennaro, M.L., Eisenberg, D.(2002) Protein Sci 11: 2887-2893
- PubMed: 12441386
- DOI: https://doi.org/10.1110/ps.0219002
- Primary Citation of Related Structures:
1LMI - PubMed Abstract:
MPT63 is a small, major secreted protein of unknown function from Mycobacterium tuberculosis that has been shown to have immunogenic properties and has been implicated in virulence. A BLAST search identified that MPT63 has homologs only in other mycobacteria, and is therefore mycobacteria specific. As MPT63 is a secreted protein, mycobacteria specific, and implicated in virulence, MPT63 is an attractive drug target against the deadliest infectious disease, tuberculosis (TB). As part of the TB Structural Genomics Consortium, the X-ray crystal structure of MPT63 was determined to 1.5-Angstrom resolution with the hope of yielding functional information about MPT63. The structure of MPT63 is an antiparallel beta-sandwich immunoglobulin-like fold, with the unusual feature of the first beta-strand of the protein forming a parallel addition to the small antiparallel beta-sheet. MPT63 has weak structural similarity to many proteins with immunoglobulin folds, in particular, Homo sapiens beta2-adaptin, bovine arrestin, and Yersinia pseudotuberculosis invasin. Although the structure of MPT63 gives no conclusive evidence to its function, structural similarity suggests that MPT63 could be involved in cell-host interactions to facilitate endocytosis/phagocytosis.
Organizational Affiliation:
Howard Hughes Medical Institute, University of California at Los Angeles-DOE, Center for Genomics and Proteomics, Los Angeles 90095, USA.