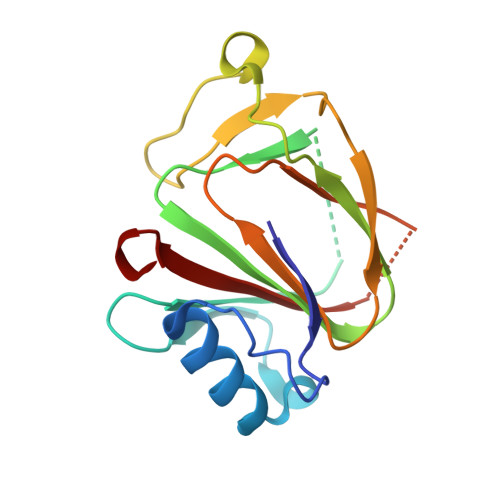
Crystal structure of the bacterial YhcH protein indicates a role in sialic acid catabolism.
Teplyakov, A., Obmolova, G., Toedt, J., Galperin, M.Y., Gilliland, G.L.(2005) J Bacteriol 187: 5520-5527
- PubMed: 16077096
- DOI: https://doi.org/10.1128/JB.187.16.5520-5527.2005
- Primary Citation of Related Structures:
1JOP, 1S4C - PubMed Abstract:
The yhcH gene is part of the nan operon in bacteria that encodes proteins involved in sialic acid catabolism. Determination of the crystal structure of YhcH from Haemophilus influenzae was undertaken as part of a structural genomics effort in order to assist with the functional assignment of the protein. The structure was determined at 2.2-A resolution by multiple-wavelength anomalous diffraction. The protein fold is a variation of the double-stranded beta-helix. Two antiparallel beta-sheets form a funnel opened at one side, where a putative active site contains a copper ion coordinated to the side chains of two histidine and two carboxylic acid residues. A comparison to other proteins with a similar fold and analysis of the genomic context suggested that YhcH may be a sugar isomerase involved in processing of exogenous sialic acid.
Organizational Affiliation:
Center for Advanced Research in Biotechnology, University of Maryland Biotechnology Institute and National Institute of Standards and Technology, Rockville, Maryland, USA. ATeplyak@cntus.jnj.com