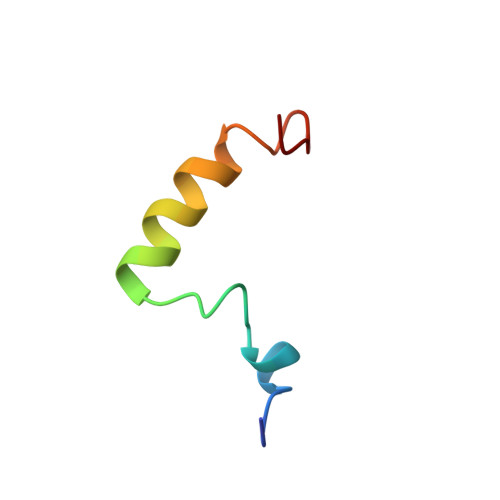
Structure of human parathyroid hormone 1-37 in solution.
Marx, U.C., Austermann, S., Bayer, P., Adermann, K., Ejchart, A., Sticht, H., Walter, S., Schmid, F.X., Jaenicke, R., Forssmann, W.G.(1995) J Biol Chem 270: 15194-15202
- PubMed: 7797503
- DOI: https://doi.org/10.1074/jbc.270.25.15194
- Primary Citation of Related Structures:
1HPH - PubMed Abstract:
Human parathyroid hormone (hPTH), amino acids Ser1 to Leu37, is biologically active with respect to both receptor binding and activation of adenylate cyclase to influence the serum calcium concentration. It induces DNA synthesis via an unknown signal pathway. We investigated the structure of hPTH(1-37) in H2O/buffer solution under near physiological conditions, that is pH 6.0 and 270 mM salt, by circular dichroism, ultracentrifugation, nuclear magnetic resonance spectroscopy, and molecular dynamics calculations. Complete sequence specific assignments of all 1H resonances were performed by using 1H two-dimensional NMR measurements (double quantum-filtered correlated spectroscopy, nuclear Overhauser effect spectroscopy (NOESY), and total correlation spectroscopy with suppression of NOESY-type cross-peaks spectra). hPTH(1-37) obtained helical structure and showed hydrophobic interactions defining a tertiary structure. The NH2-terminal four amino acids of hPTH(1-37) did not show a stable conformation. Evidence for an alpha-helical region between Ile5 and Asn10 was found. This region was followed by a flexible link (Gly12, Lys13) and a well defined turn region, His14 to Ser17. The latter was stabilized by hydrophobic interactions between Trp23 and Leu15. Ser17 through at least Leu28 formed an alpha-helix. Arg20 and Lys27 were involved in the core built by His14 to Ser17. Unrestrained molecular dynamics simulations indicated that the structure was stable on the 200 ps time scale.
Organizational Affiliation:
Lehrstuhl für Biochemie, Universität Bayreuth, Federal Republic of Germany.