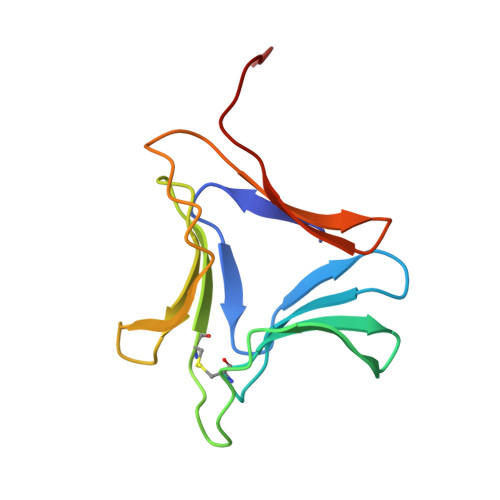
Crystal structure of a dimeric mannose-specific agglutinin from garlic: quaternary association and carbohydrate specificity.
Chandra, N.R., Ramachandraiah, G., Bachhawat, K., Dam, T.K., Surolia, A., Vijayan, M.(1999) J Mol Biol 285: 1157-1168
- PubMed: 9887270
- DOI: https://doi.org/10.1006/jmbi.1998.2353
- Primary Citation of Related Structures:
1BWU - PubMed Abstract:
A mannose-specific agglutinin, isolated from garlic bulbs, has been crystallized in the presence of a large excess of alpha-d-mannose, in space group C2 and cell dimensions, a=203.24, b=43.78, c=79.27 A, beta=112.4 degrees, with two dimers in the asymmetric unit. X-ray diffraction data were collected up to a nominal resolution of 2.4 A and the structure was solved by molecular replacement. The structure, refined to an R-factor of 22.6 % and an Rfree of 27.8 % reveals a beta-prism II fold, similar to that in the snowdrop lectin, comprising three antiparallel four-stranded beta-sheets arranged as a 12-stranded beta-barrel, with an approximate internal 3-fold symmetry. This agglutinin is, however, a dimer unlike snowdrop lectin which exists as a tetramer, despite a high degree of sequence similarity between them. A comparison of the two structures reveals a few substitutions in the garlic lectin which stabilise it into a dimer and prevent tetramer formation. Three mannose molecules have been identified on each subunit. In addition, electron density is observed for another possible mannose molecule per dimer resulting in a total of seven mannose molecules in each dimer. Although the mannose binding sites and the overall structure are similar in the subunits of snowdrop and garlic lectin, their specificities to glycoproteins such as GP120 vary considerably. These differences appear, in part, to be a direct consequence of the differences in oligomerisation, implying that variation in quaternary association may be a mode of achieving oligosaccharide specificity in bulb lectins.
Organizational Affiliation:
Molecular Biophysics Unit, Indian Institute of Science, Bangalore, 560 012, India.