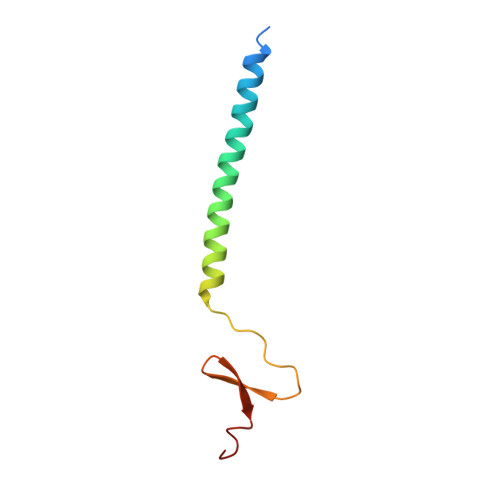
Structure of bacteriophage T4 fibritin M: a troublesome packing arrangement.
Strelkov, S.V., Tao, Y., Shneider, M.M., Mesyanzhinov, V.V., Rossmann, M.G.(1998) Acta Crystallogr D Biol Crystallogr 54: 805-816
- PubMed: 9757094
- DOI: https://doi.org/10.1107/s0907444997018878
- Primary Citation of Related Structures:
1AVY - PubMed Abstract:
Fibritin, a 52 kDa product of bacteriophage T4 gene wac, forms 530 A long fibers, named whiskers, that attach to the phage neck and perform a helper function during phage assembly. Fibritin is a homotrimer, with its predominant central domain consisting of 12 consecutive alpha-helical coiled-coil segments linked together by loops. The central domain is flanked by small globular domains at both ends. Fibritin M is a genetically engineered fragment of the wild type and contains 74 amino-acid residues corresponding to the last coiled-coil segment and the complete carboxy-terminal domain. The crystals of fibritin M belong to the rare space group P3 with three crystallographically independent trimers in the unit cell. The structure has been established at 1.85 A resolution by combining molecular and isomorphous replacement techniques. One of the two heavy-atom derivatives used was gaseous xenon. A substantial fraction of residues in each independent trimer is disordered to various extents in proportion to the lack of restraints on the molecules provided by the lattice contacts. Accurate modeling of the solvent present in the crystals was crucial for achieving good agreement with experimental data.
Organizational Affiliation:
Department of Biological Sciences, Purdue University, West Lafayette, IN 47907-1392, USA.