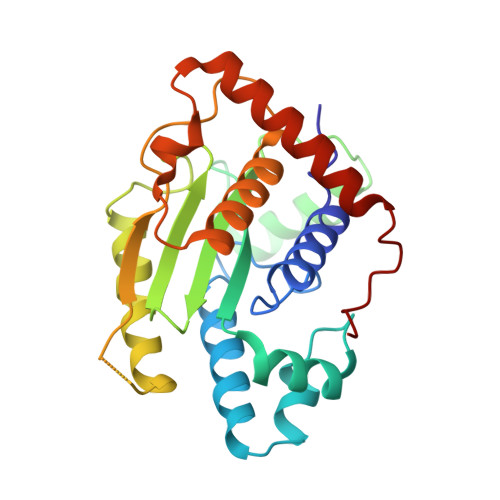
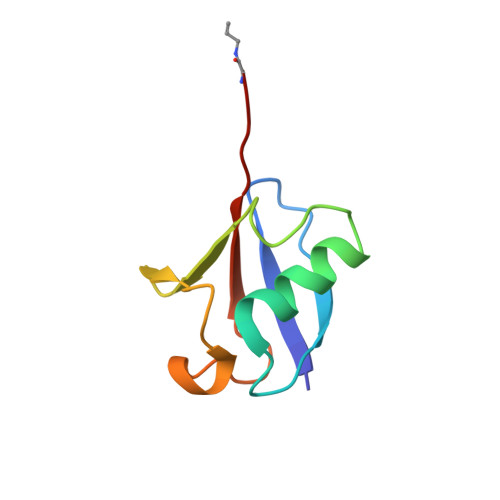
The Two Deubiquitinating Enzymes fromChlamydia trachomatisHave Distinct Ubiquitin Recognition Properties.
Hausman, J.M., Kenny, S., Iyer, S., Babar, A., Qiu, J., Fu, J., Luo, Z.Q., Das, C.(2020) Biochemistry 59: 1604-1617
- PubMed: 32275137
- DOI: https://doi.org/10.1021/acs.biochem.9b01107
- Primary Citation of Related Structures:
6MRN, 6OAM - PubMed Abstract:
Chlamydia trachomatis is the cause of several diseases such as sexually transmitted urogenital disease and ocular trachoma. The pathogen contains a small genome yet, upon infection, expresses two enzymes with deubiquitinating activity, termed ChlaDUB1 and ChlaDUB2, presumed to have redundant deubiquitinase (DUB) function because of the similarity of the primary structure of their catalytic domain. Previous studies have led to structural characterization of the enzymatic properties of ChlaDUB1; however, ChlaDUB2 has yet to be investigated thoroughly. In this study, we investigated the deubiquitinase properties of ChlaDUB2 and compared them to those of ChlaDUB1. This revealed a distinct difference in hydrolytic activity with regard to di- and polyubiquitin chains while showing similar ability to cleave a monoubiquitin-based substrate, ubiquitin aminomethylcoumarin (Ub-AMC). ChlaDUB2 was unable to cleave a diubiquitin substrate efficiently, whereas ChlaDUB1 could rapidly hydrolyze this substrate like a prototypical prokaryotic DUB, SdeA. With polyubiquitinated green fluorescent protein substrate (GFP-Ub n ), whereas ChlaDUB1 efficiently disassembled the polyubiquitin chains into the monoubiquitin product, the deubiquitination activity of ChlaDUB2, while showing depletion of the substrate, did not produce appreciable levels of the monoubiquitin product. We report the structures of a catalytic construct of ChlaDUB2 and its complex with ubiquitin propargyl amide. These structures revealed differences in residues involved in substrate recognition between the two Chlamydia DUBs. On the basis of the structures, we conclude that the distal ubiquitin binding is equivalent between the two DUBs, consistent with the Ub-AMC activity result. Therefore, the difference in activity with longer ubiquitinated substrates may be due to the differential recognition of these substrates involving additional ubiquitin binding sites.
Organizational Affiliation:
Department of Chemistry, Purdue University, 560 Oval Drive, West Lafayette, Indiana 47907, United States.