Molecular mechanisms behind DAPK regulation: how phosphorylation switches work
Huart, A.-S., Simon, B., Lubner, J., Mertens, H.D.T., Temmerman, K., Hoffmann, J.-E., Svergun, D.I., Schwartz, D., Schultz, C., Wilmanns, M.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Death-associated protein kinase 1 | 337 | Homo sapiens | Mutation(s): 2 Gene Names: DAPK1, DAPK EC: 2.7.11.1 | 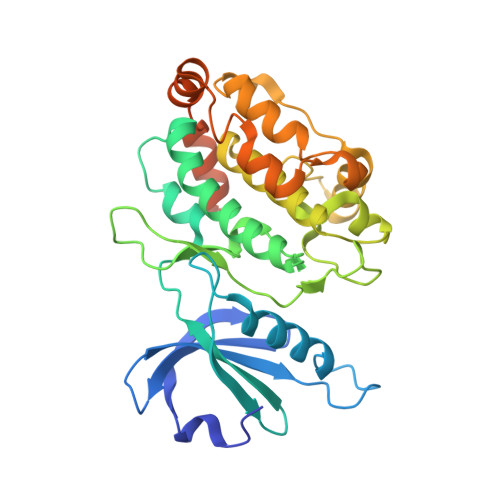 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P53355 (Homo sapiens) Explore P53355 Go to UniProtKB: P53355 | |||||
PHAROS: P53355 GTEx: ENSG00000196730 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P53355 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 46.993 | α = 90 |
| b = 75.82 | β = 90 |
| c = 93.511 | γ = 90 |
| Software Name | Purpose |
|---|---|
| MxCuBE | data collection |
| XDS | data reduction |
| Aimless | data scaling |
| Coot | model building |
| PHENIX | refinement |
| PHASER | phasing |