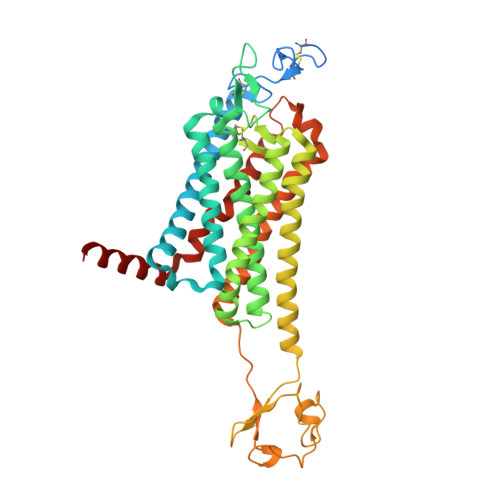
Crystal structure of the Frizzled 4 receptor in a ligand-free state.
Yang, S., Wu, Y., Xu, T.H., de Waal, P.W., He, Y., Pu, M., Chen, Y., DeBruine, Z.J., Zhang, B., Zaidi, S.A., Popov, P., Guo, Y., Han, G.W., Lu, Y., Suino-Powell, K., Dong, S., Harikumar, K.G., Miller, L.J., Katritch, V., Xu, H.E., Shui, W., Stevens, R.C., Melcher, K., Zhao, S., Xu, F.(2018) Nature 560: 666-670
- PubMed: 30135577
- DOI: https://doi.org/10.1038/s41586-018-0447-x
- Primary Citation of Related Structures:
6BD4 - PubMed Abstract:
Frizzled receptors (FZDs) are class-F G-protein-coupled receptors (GPCRs) that function in Wnt signalling and are essential for developing and adult organisms 1,2 . As central mediators in this complex signalling pathway, FZDs serve as gatekeeping proteins both for drug intervention and for the development of probes in basic and in therapeutic research. Here we present an atomic-resolution structure of the human Frizzled 4 receptor (FZD4) transmembrane domain in the absence of a bound ligand. The structure reveals an unusual transmembrane architecture in which helix VI is short and tightly packed, and is distinct from all other GPCR structures reported so far. Within this unique transmembrane fold is an extremely narrow and highly hydrophilic pocket that is not amenable to the binding of traditional GPCR ligands. We show that such a pocket is conserved across all FZDs, which may explain the long-standing difficulties in the development of ligands for these receptors. Molecular dynamics simulations on the microsecond timescale and mutational analysis uncovered two coupled, dynamic kinks located at helix VII that are involved in FZD4 activation. The stability of the structure in its ligand-free form, an unfavourable pocket for ligand binding and the two unusual kinks on helix VII suggest that FZDs may have evolved a novel ligand-recognition and activation mechanism that is distinct from that of other GPCRs.
Organizational Affiliation:
iHuman Institute, ShanghaiTech University, Shanghai, China.