6AWN
X-ray structure of the S439T human serotonin transporter complexed with paroxetine at the central site
- PDB DOI: https://doi.org/10.2210/pdb6AWN/pdb
- Classification: TRANSPORT PROTEIN
- Organism(s): Homo sapiens, Mus musculus
- Expression System: Homo sapiens, Spodoptera frugiperda
- Mutation(s): Yes
- Membrane Protein: Yes PDBTMMemProtMDmpstruc
- Deposited: 2017-09-06 Released: 2017-10-25
- Funding Organization(s): National Institutes of Health/National Institute of Mental Health (NIH/NIMH)
Experimental Data Snapshot
- Method: X-RAY DIFFRACTION
- Resolution: 3.62 Å
- R-Value Free: 0.263
- R-Value Work: 0.256
- R-Value Observed: 0.256
This is version 1.8 of the entry. See complete history.
Macromolecules
Find similar proteins by:
(by identity cutoff) | 3D Structure
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Sodium-dependent serotonin transporter | 549 | Homo sapiens | Mutation(s): 4 Gene Names: SLC6A4, HTT, SERT Membrane Entity: Yes | 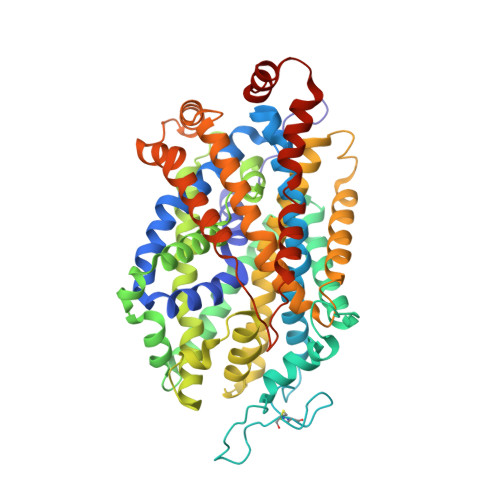 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P31645 (Homo sapiens) Explore P31645 Go to UniProtKB: P31645 | |||||
PHAROS: P31645 GTEx: ENSG00000108576 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P31645 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by:
(by identity cutoff) | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| 8B6 antibody FAB heavy chain | 229 | Mus musculus | Mutation(s): 0 Membrane Entity: Yes | 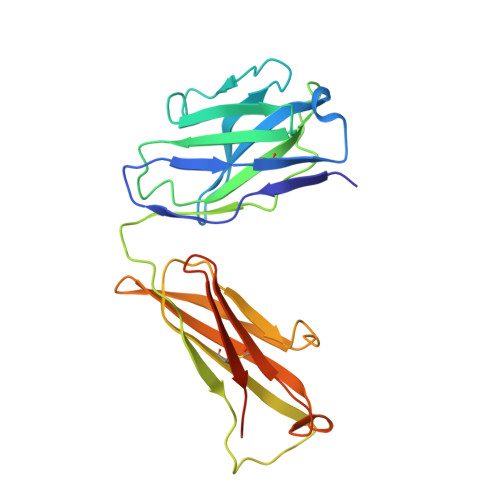 | |
UniProt | |||||
Find proteins for A0A0F7R1P3 (Mus musculus) Explore A0A0F7R1P3 Go to UniProtKB: A0A0F7R1P3 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A0F7R1P3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by:
(by identity cutoff) | 3D Structure
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| 8B6 antibody FAB light chain | 214 | Mus musculus | Mutation(s): 0 Membrane Entity: Yes | 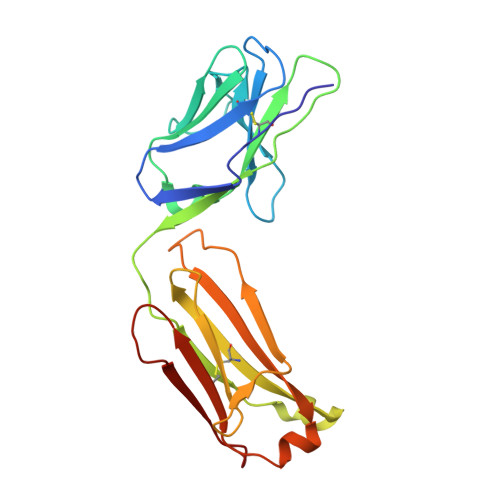 | |
UniProt | |||||
Find proteins for A0A0F7R5U8 (Mus musculus) Explore A0A0F7R5U8 Go to UniProtKB: A0A0F7R5U8 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A0F7R5U8 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Small Molecules
| Ligands 6 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| LMT Query on LMT | F [auth A] | DODECYL-BETA-D-MALTOSIDE C24 H46 O11 NLEBIOOXCVAHBD-QKMCSOCLSA-N |  | ||
| CLR Query on CLR | E [auth A] | CHOLESTEROL C27 H46 O HVYWMOMLDIMFJA-DPAQBDIFSA-N |  | ||
| 8PR Query on 8PR | H [auth A] | Paroxetine C19 H20 F N O3 AHOUBRCZNHFOSL-YOEHRIQHSA-N |  | ||
| NAG Query on NAG | D [auth A], G [auth A] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| CL Query on CL | I [auth A] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| NA Query on NA | J [auth A] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
Experimental Data & Validation
Experimental Data
- Method: X-RAY DIFFRACTION
- Resolution: 3.62 Å
- R-Value Free: 0.263
- R-Value Work: 0.256
- R-Value Observed: 0.256
- Space Group: C 2 2 21
Unit Cell:
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 129.15 | α = 90 |
| b = 162.84 | β = 90 |
| c = 140.82 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| XSCALE | data scaling |
| PHASER | phasing |
Entry History & Funding Information
Deposition Data
- Released Date: 2017-10-25 Deposition Author(s): Coleman, J.A., Gouaux, E.
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of Mental Health (NIH/NIMH) | United States | 5R37MH070039 |
Revision History (Full details and data files)
- Version 1.0: 2017-10-25
Type: Initial release - Version 1.1: 2017-11-22
Changes: Database references - Version 1.2: 2017-12-06
Changes: Author supporting evidence - Version 1.3: 2018-01-31
Changes: Database references - Version 1.4: 2018-02-14
Changes: Database references - Version 1.5: 2018-02-21
Changes: Database references - Version 1.6: 2019-11-27
Changes: Author supporting evidence - Version 1.7: 2020-07-29
Type: Remediation
Reason: Carbohydrate remediation
Changes: Data collection, Derived calculations, Structure summary - Version 1.8: 2023-10-04
Changes: Data collection, Database references, Refinement description, Structure summary













