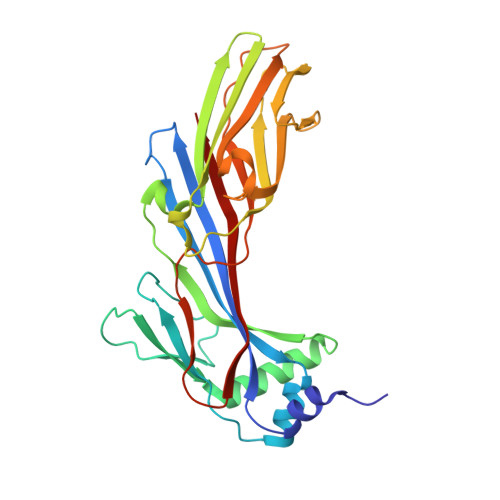
SGIP1 dimerizes via intermolecular disulfide bond in mu HD domain during cellular endocytosis.
Zhang, Y., Feng, Y., Xin, Y., Liu, X.(2018) Biochem Biophys Res Commun 505: 99-105
- PubMed: 30236986
- DOI: https://doi.org/10.1016/j.bbrc.2018.09.075
- Primary Citation of Related Structures:
6A9Y - PubMed Abstract:
Along with its homologs FCHo1 and FCHo2, SGIP1 plays an important role in clathrin-mediated endocytosis. The highly conserved C-terminal μHD domains in these proteins are the critical regions interacting with adapter molecules such as Eps15. The crystal structure of μHD domain of SGIP1 has been reported previously. In this study, we found that μHD domain of SGIP1 is capable of forming a stable dimer by an intermolecular disulfide bond formed by C632 in our crystal structure. The mutational study of C632 revealed that this residue is important for the function of SGIP1 during cellular endocytosis. Our study revealed a new dimerization and/or oligomerization manner in theses adaptor proteins, which is a critical prerequisite for their proper function.
Organizational Affiliation:
State Key Laboratory of Medicinal Chemical Biology, Department of Biochemistry and Molecular Biology, College of Life Sciences, Nankai University, Tianjin, 300071, China.