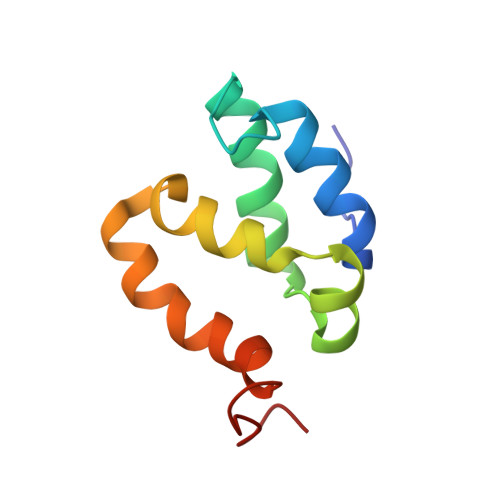
1H, 15N and 13C assignments of the N-terminal domain of the Mediator complex subunit MED26.
Peruzzini, R., Lens, Z., Verger, A., Dewitte, F., Ferreira, E., Baert, J.L., Villeret, V., Landrieu, I., Cantrelle, F.X.(2016) Biomol NMR Assign 10: 233-236
- PubMed: 26861138
- DOI: https://doi.org/10.1007/s12104-016-9673-z
- Primary Citation of Related Structures:
5ODD - PubMed Abstract:
MED26 is a subunit of the Mediator, a very large complex involved in regulation of gene transcription by RNA Polymerase II. MED26 regulates the switch between initiation and elongation phases of the transcription. This function requires interaction of its N-terminal domain (NTD) with several protein partners implicated in transcriptional regulation. Molecular details of the structure and interaction mode of MED26 NTD would improve understanding of this complex regulation. As a first step towards structural characterization, sequence specific (1)H, (13)C and (15)N assignments for MED26 NTD was performed based on Nuclear Magnetic Resonance spectroscopy. TALOS+ analysis of the chemical shifts data revealed a domain solely composed of helices. Assignments will be further used to solve NMR structure and dynamics of MED26 NTD and investigate the molecular details of its interaction with protein partners.
Organizational Affiliation:
Lille University, UMR8576, CNRS, 59000, Lille, France.