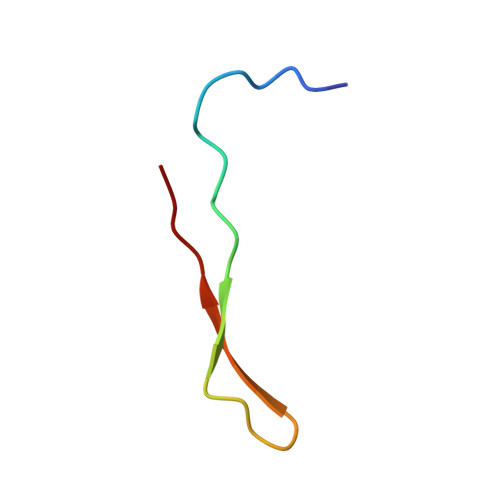
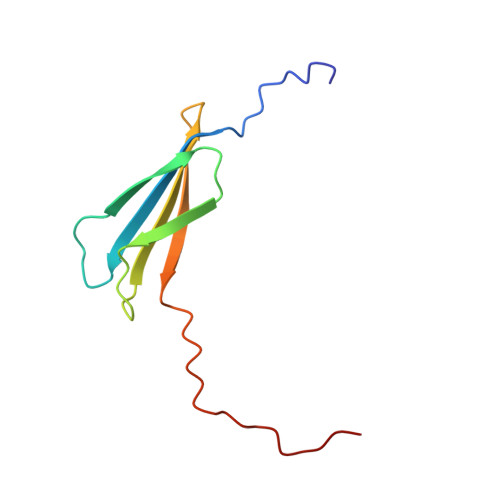
The Interaction between the Third Type III Domain from Fibronectin and Anastellin Involves beta-Strand Exchange.
Stine, J.M., Ahl, G.J.H., Schlenker, C., Rusnac, D.V., Briknarova, K.(2017) Biochemistry 56: 4667-4675
- PubMed: 28820240
- DOI: https://doi.org/10.1021/acs.biochem.7b00633
- Primary Citation of Related Structures:
5J6Z - PubMed Abstract:
Anastellin is a small recombinant fragment derived from the extracellular matrix protein fibronectin; it comprises the first type III (FN3) domain without the two N-terminal β-strands. It inhibits angiogenesis, tumor growth, and metastasis in mouse models and requires endogenous fibronectin for its in vivo anti-angiogenic activity. It binds to fibronectin in vitro and converts the soluble protein to insoluble fibrils that structurally and functionally resemble fibronectin fibrils deposited in the extracellular matrix by cells. Anastellin binds to several FN3 domains in fibronectin, but how it interacts with these domains and why the interactions lead to aggregation of fibronectin are not well understood. In this work, we investigated the interaction between anastellin and the third FN3 domain (3FN3) from fibronectin. We show that anastellin binds with high affinity to a peptide comprising the two N-terminal β-strands from 3FN3, and we present here the structure of the resulting complex. The peptide and anastellin form a composite FN3 domain, with the two N-terminal β-strands from 3FN3 bound in place of the two β-strands that are missing in anastellin. We also demonstrate using disulfide cross-linking that a similar interaction involving the two N-terminal β-strands of 3FN3 occurs when intact 3FN3 binds to anastellin. 3FN3 adopts a compact globular fold in solution, and to interact with anastellin in a manner consistent with our data, it has to open up and expose a β-strand edge that is not accessible in the context of the folded domain.
Organizational Affiliation:
Department of Chemistry and Biochemistry, University of Montana , Missoula, Montana 59812, United States.