Molecular Architecture of SF3b and Structural Consequences of Its Cancer-Related Mutations.
Cretu, C., Schmitzova, J., Ponce-Salvatierra, A., Dybkov, O., De Laurentiis, E.I., Sharma, K., Will, C.L., Urlaub, H., Luhrmann, R., Pena, V.(2016) Mol Cell 64: 307-319
- PubMed: 27720643
- DOI: https://doi.org/10.1016/j.molcel.2016.08.036
- Primary Citation of Related Structures:
5HY7, 5IFE - PubMed Abstract:
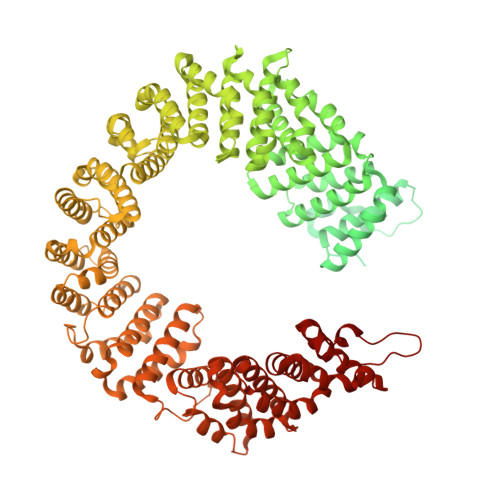
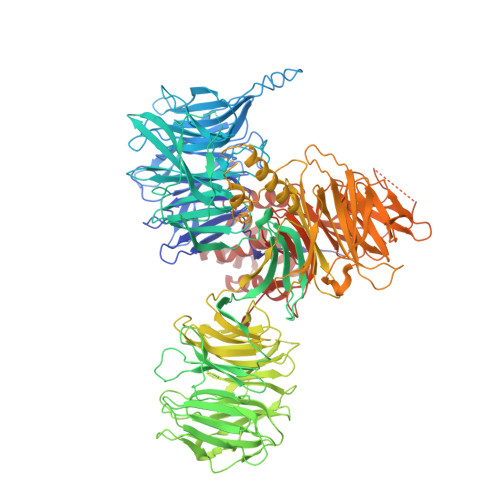
SF3b is a heptameric protein complex of the U2 small nuclear ribonucleoprotein (snRNP) that is essential for pre-mRNA splicing. Mutations in the largest SF3b subunit, SF3B1/SF3b155, are linked to cancer and lead to alternative branch site (BS) selection. Here we report the crystal structure of a human SF3b core complex, revealing how the distinctive conformation of SF3b155's HEAT domain is maintained by multiple contacts with SF3b130, SF3b10, and SF3b14b. Protein-protein crosslinking enabled the localization of the BS-binding proteins p14 and U2AF65 within SF3b155's HEAT-repeat superhelix, which together with SF3b14b forms a composite RNA-binding platform. SF3b155 residues, the mutation of which leads to cancer, contribute to the tertiary structure of the HEAT superhelix and its surface properties in the proximity of p14 and U2AF65. The molecular architecture of SF3b reveals the spatial organization of cancer-related SF3b155 mutations and advances our understanding of their effects on SF3b structure and function.
Organizational Affiliation:
Research Group Macromolecular Crystallography, Max Planck Institute for Biophysical Chemistry, Am Fassberg 11, 37077 Göttingen, Germany.