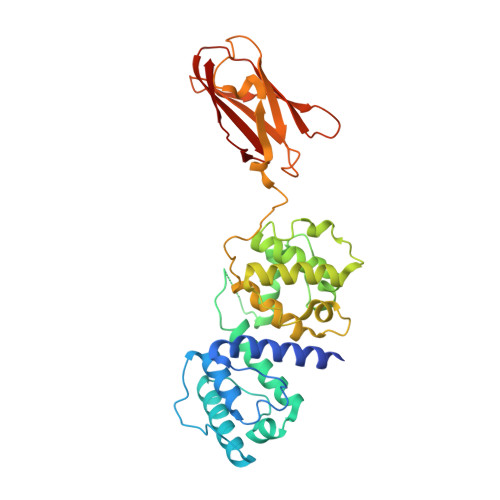
Crystal Structure of the Filamin N-Terminal Region Reveals a Hinge between the Actin Binding and First Repeat Domains
Sawyer, G.M., Sutherland-Smith, A.J.(2012) J Mol Biol 424: 240
- PubMed: 23036857
- DOI: https://doi.org/10.1016/j.jmb.2012.09.016
- Primary Citation of Related Structures:
4B7L - PubMed Abstract:
The filamin proteins cross-link F-actin and interact with protein partners to integrate both extracellular and intracellular signalling events with the cytoskeleton and to provide mechanoprotection and sensing to cells. The filamins are large, flexible, multi-domain homodimers with the interactions between domains important for protein function. The crystal structure of the N-terminal region of filamin B, containing the actin binding domain (ABD) and the first filamin repeat (FR1) domain, reveals an extended two-domain conformation with no interaction between the ABD and FR1 other than the connecting linker region. The two FLNB347 structures in the crystallographic asymmetric unit exhibit differing relative domain orientations providing the first high-resolution structural characterisation of a filamin inter-domain conformational change. The structure reveals a new hinge in the linker region between ABD and FR1 that is ideally positioned to orient the ABD for actin binding and adds to the previously described hinge regions, hinge 1 (between repeats 15 and 16) and hinge 2 (repeats 23 and 24), providing an additional mechanism by which filamin can exhibit inter-domain flexibility. The extended structure, with the absence of interactions between the domains, implies that any conformational rearrangements required for actin binding by the ABD, as observed for homologous proteins, can freely occur without being influenced by FR1. The ABD retains its previously observed compact conformation. FR1 exhibits a filamin immunoglobulin-like domain fold with a closed C-D β-strand groove, in contrast to filamin repeats that bind protein partners with this region of the domain surface.
Organizational Affiliation:
Institute of Molecular Biosciences, Massey University, Private Bag 11222, Palmerston North 4442, New Zealand.