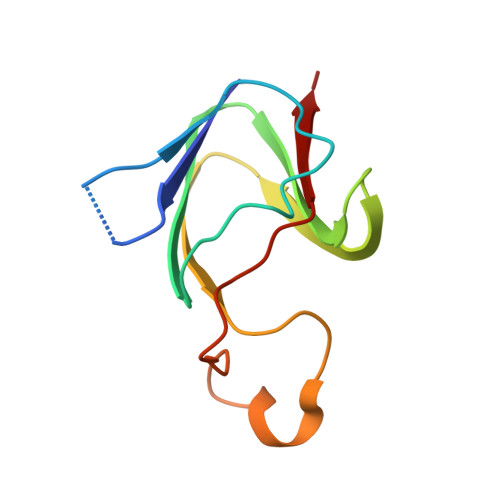
Exploring the limits of sequence and structure in a variant betagamma-crystallin domain of the protein absent in melanoma-1 (AIM1).
Aravind, P., Wistow, G., Sharma, Y., Sankaranarayanan, R.(2008) J Mol Biol 381: 509-518
- PubMed: 18582473
- DOI: https://doi.org/10.1016/j.jmb.2008.06.019
- Primary Citation of Related Structures:
3CW3 - PubMed Abstract:
Betagamma-crystallins belong to a superfamily of proteins in prokaryotes and eukaryotes that are based on duplications of a characteristic, highly conserved Greek key motif. Most members of the superfamily in vertebrates are structural proteins of the eye lens that contain four motifs arranged as two structural domains. Absent in melanoma 1 (AIM1), an unusual member of the superfamily whose expression is associated with suppression of malignancy in melanoma, contains 12 betagamma-crystallin motifs in six domains. Some of these motifs diverge considerably from the canonical motif sequence. AIM1g1, the first betagamma-crystallin domain of AIM1, is the most variant of betagamma-crystallin domains currently known. In order to understand the limits of sequence variation on the structure, we report the crystal structure of AIM1g1 at 1.9 A resolution. Despite having changes in key residues, the domain retains the overall betagamma-crystallin fold. The domain also contains an unusual extended surface loop that significantly alters the shape of the domain and its charge profile. This structure illustrates the resilience of the betagamma fold to considerable sequence changes and its remarkable ability to adapt for novel functions.
Organizational Affiliation:
Centre for Cellular and Molecular Biology, Uppal Road, Hyderabad-500007, India.