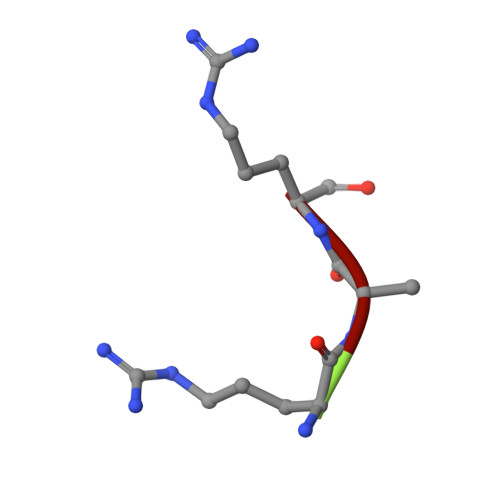
Structure-based dissection of the active site chemistry of leukotriene a4 hydrolase: implications for m1 aminopeptidases and inhibitor design.
Tholander, F., Muroya, A., Roques, B.P., Fournie-Zaluski, M.C., Thunnissen, M.M., Haeggstrom, J.Z.(2008) Chem Biol 15: 920-929
- PubMed: 18804029
- DOI: https://doi.org/10.1016/j.chembiol.2008.07.018
- Primary Citation of Related Structures:
2R59, 3B7R, 3B7S, 3B7T, 3B7U - PubMed Abstract:
M1 aminopeptidases comprise a large family of biologically important zinc enzymes. We show that peptide turnover by the M1 prototype, leukotriene A4 hydrolase/aminopeptidase, involves a shift in substrate position associated with exchange of zinc coordinating groups, while maintaining the overall coordination geometry. The transition state is stabilized by residues conserved among M1 members and in the final reaction step, Glu-296 of the canonical zinc binding HEXXH motif shuffles a proton from the hydrolytic water to the leaving group. Tripeptide substrates bind along the conserved GXMEN motif, precisely occupying the distance between Glu-271 and Arg-563, whereas the Arg specificity is governed by a narrow S1 pocket capped with Asp-375. Our data provide detailed insights to the active site chemistry of M1 aminopeptidases and will aid in the development of novel enzyme inhibitors.
Organizational Affiliation:
Department of Medical Biochemistry and Biophysics, Division of Chemistry II, Karolinska Institute, Stockholm, Sweden.