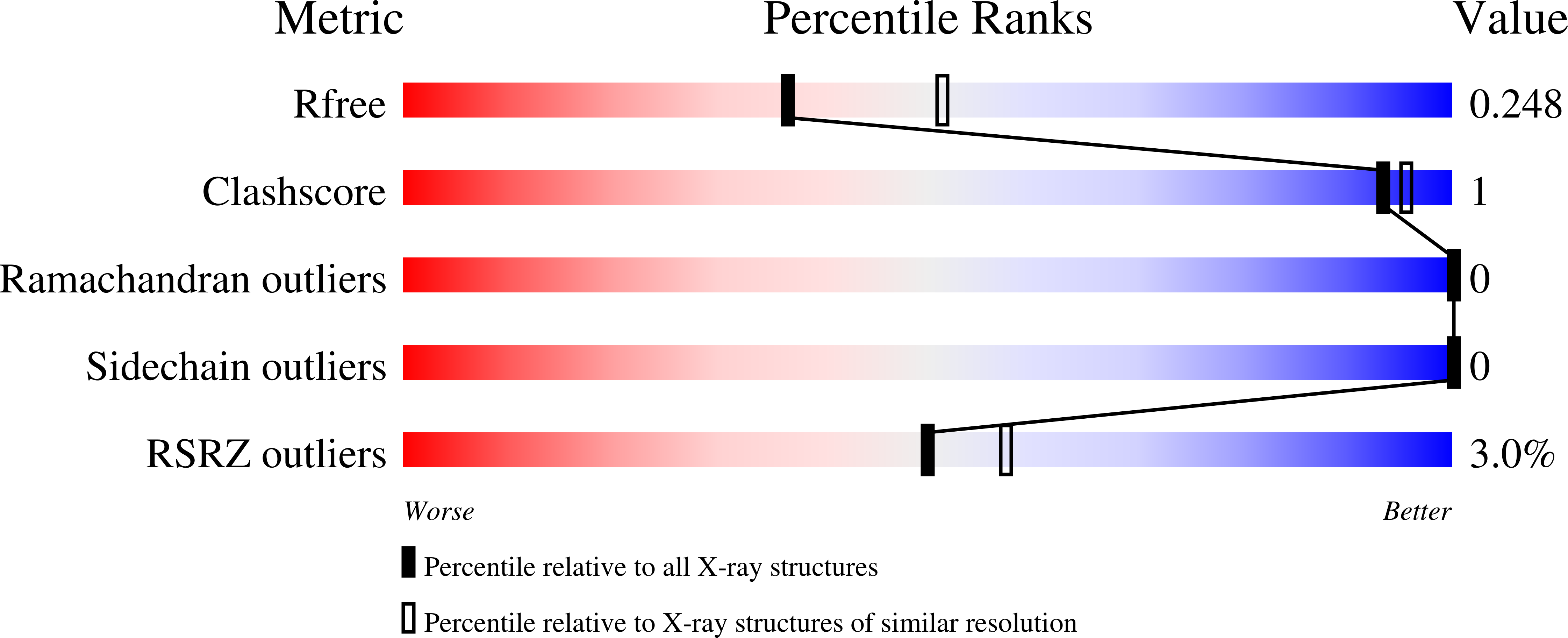
Dcp1 Forms Asymmetric Trimers to Assemble Into Active Mrna Decapping Complexes in Metazoa.
Tritschler, F., Braun, J.E., Motz, C., Igreja, C., Haas, G., Truffault, V., Izaurralde, E., Weichenrieder, O.(2009) Proc Natl Acad Sci U S A 106: 21591
- PubMed: 19966221
- DOI: https://doi.org/10.1073/pnas.0909871106
- Primary Citation of Related Structures:
2WX3, 2WX4 - PubMed Abstract:
DCP1 stimulates the decapping enzyme DCP2, which removes the mRNA 5' cap structure committing mRNAs to degradation. In multicellular eukaryotes, DCP1-DCP2 interaction is stabilized by additional proteins, including EDC4. However, most information on DCP2 activation stems from studies in S. cerevisiae, which lacks EDC4. Furthermore, DCP1 orthologs from multicellular eukaryotes have a C-terminal extension, absent in fungi. Here, we show that in metazoa, a conserved DCP1 C-terminal domain drives DCP1 trimerization. Crystal structures of the DCP1-trimerization domain reveal an antiparallel assembly comprised of three kinked alpha-helices. Trimerization is required for DCP1 to be incorporated into active decapping complexes and for efficient mRNA decapping in vivo. Our results reveal an unexpected connectivity and complexity of the mRNA decapping network in multicellular eukaryotes, which likely enhances opportunities for regulating mRNA degradation.
Organizational Affiliation:
Department of Biochemistry, Max Planck Institute for Developmental Biology, Spemannstrasse 35, D-72076 Tübingen, Germany.