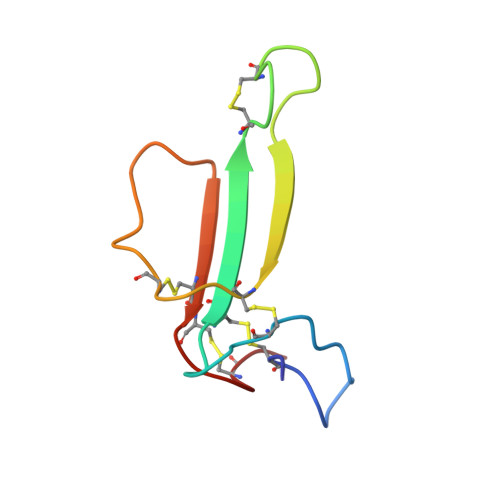
Solution structure of neuronal bungarotoxin determined by two-dimensional NMR spectroscopy: calculation of tertiary structure using systematic homologous model building, dynamical simulated annealing, and restrained molecular dynamics.
Sutcliffe, M.J., Dobson, C.M., Oswald, R.E.(1992) Biochemistry 31: 2962-2970
- PubMed: 1550821
- DOI: https://doi.org/10.1021/bi00126a017
- Primary Citation of Related Structures:
2NBT - PubMed Abstract:
Neuronal bungarotoxin has previously been shown, using two-dimensional 1H NMR spectroscopy, to have a triple-stranded antiparallel beta-sheet structure which dimerizes in solution [Oswald, R.E., Sutcliffe, M.J., Bamberger, M., Loring, R.H., Braswell, E., & Dobson, C.M. (1991) Biochemistry 30, 4901-4909]. In this paper, structural calculations are described which use the 582 experimentally measured NOE restraints in conjunction with 27 phi-angle restraints from J-value measurements. The positions of the N-terminal region and C-terminal region were poorly defined in the calculated structures with respect to the remainder of the structure. The region of the structure containing the triple-stranded beta-sheet was, however, well defined and similar to that found in the structure of homologous alpha-bungarotoxin (45% amino acid identity). The experimental restraints did not result in a well-defined dimer interface region because of the small number of NOEs which could be identified in this region. An approach was therefore adopted which produced model structures based to varying degrees on the alpha-bungarotoxin structure. Fourteen different structures were generated in this manner and subsequently used as starting points for refinement using dynamical simulated annealing followed by restrained molecular dynamics. This approach, which combines NMR data and homologous model building, has enabled a family of structures to be proposed for the dimeric molecule. In particular, Phe49 has been identified as possibly playing an important role in dimer formation, this residue in one chain interacting with the corresponding residue in the adjacent chain.
Organizational Affiliation:
Biological NMR Centre, University of Leicester, U.K.