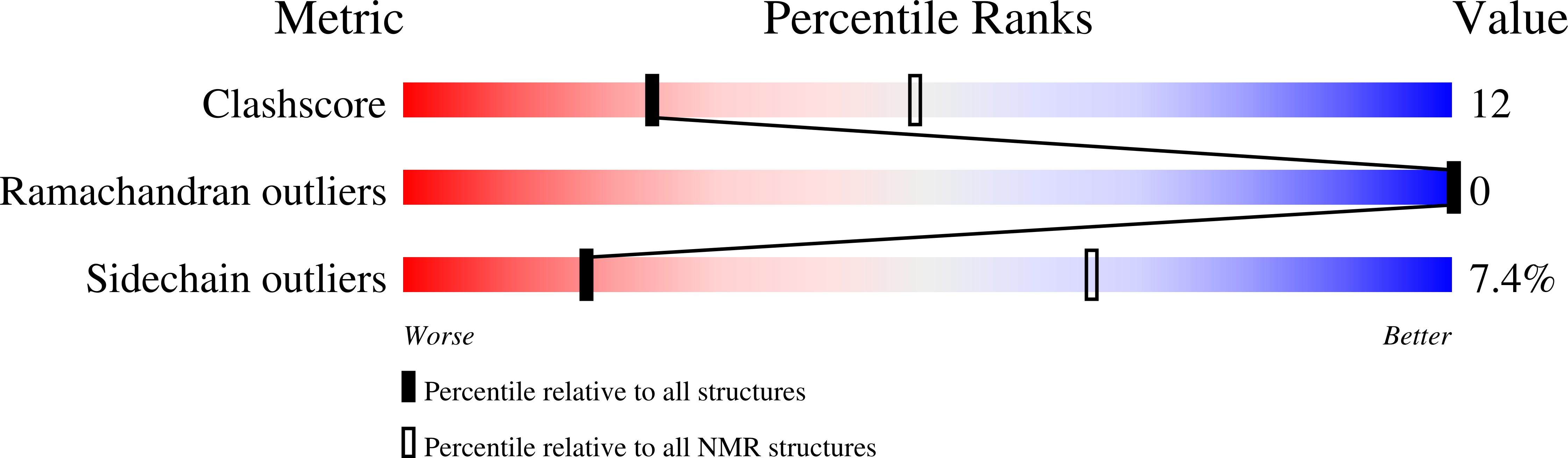Two distinct modes of ESCRT-III recognition are required for VPS4 functions in lysosomal protein targeting and HIV-1 budding
Kieffer, C., Skalicky, J.J., Morita, E., De Domenico, I., Ward, D.M., Kaplan, J., Sundquist, W.I.(2008) Dev Cell 15: 62-73
- PubMed: 18606141
- DOI: https://doi.org/10.1016/j.devcel.2008.05.014
- Primary Citation of Related Structures:
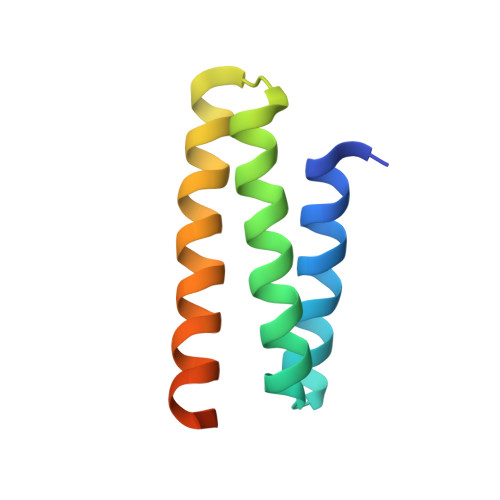
2K3W - PubMed Abstract:
The ESCRT pathway mediates membrane remodeling during enveloped virus budding, cytokinesis, and intralumenal endosomal vesicle formation. Late in the pathway, a subset of membrane-associated ESCRT-III proteins display terminal amphipathic "MIM1" helices that bind and recruit VPS4 ATPases via their MIT domains. We now report that VPS4 MIT domains also bind a second, "MIM2" motif found in a different subset of ESCRT-III subunits. The solution structure of the VPS4 MIT-CHMP6 MIM2 complex revealed that MIM2 elements bind in extended conformations along the groove between the first and third helices of the MIT domain. Mutations that block VPS4 MIT-MIM2 interactions inhibit VPS4 recruitment, lysosomal protein targeting, and HIV-1 budding. MIT-MIM2 interactions appear to be common throughout the ESCRT pathway and possibly elsewhere, and we suggest how these interactions could contribute to a mechanism in which VPS4 and ESCRT-III proteins function together to constrict the necks of budding vesicles.
Organizational Affiliation:
Department of Biochemistry, University of Utah School of Medicine, Salt Lake City, UT 84112-5650, USA.