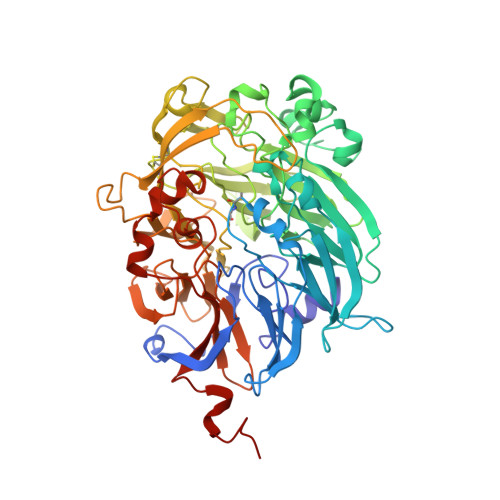
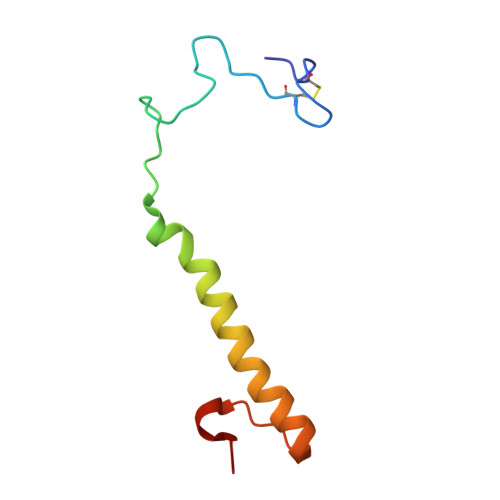
The Refined Structure of the Quinoprotein Methanol Dehydrogenase from Methylobacterium Extorquens at 1.94 A.
Ghosh, M., Anthony, C., Harlos, K., Goodwin, M.G., Blake, C.(1995) Structure 3: 177
- PubMed: 7735834
- DOI: https://doi.org/10.1016/s0969-2126(01)00148-4
- Primary Citation of Related Structures:
1H4I - PubMed Abstract:
Methanol dehydrogenase (MDH) is a bacterial periplasmic quinoprotein; it has pyrrolo-quinoline quinone (PQQ) as its prosthetic group, requires Ca2+ for activity and uses cytochrome cL as its electron acceptor. Low-resolution structures of MDH have already been determined.
Organizational Affiliation:
Laboratory of Molecular Biophysics, University of Oxford, UK.