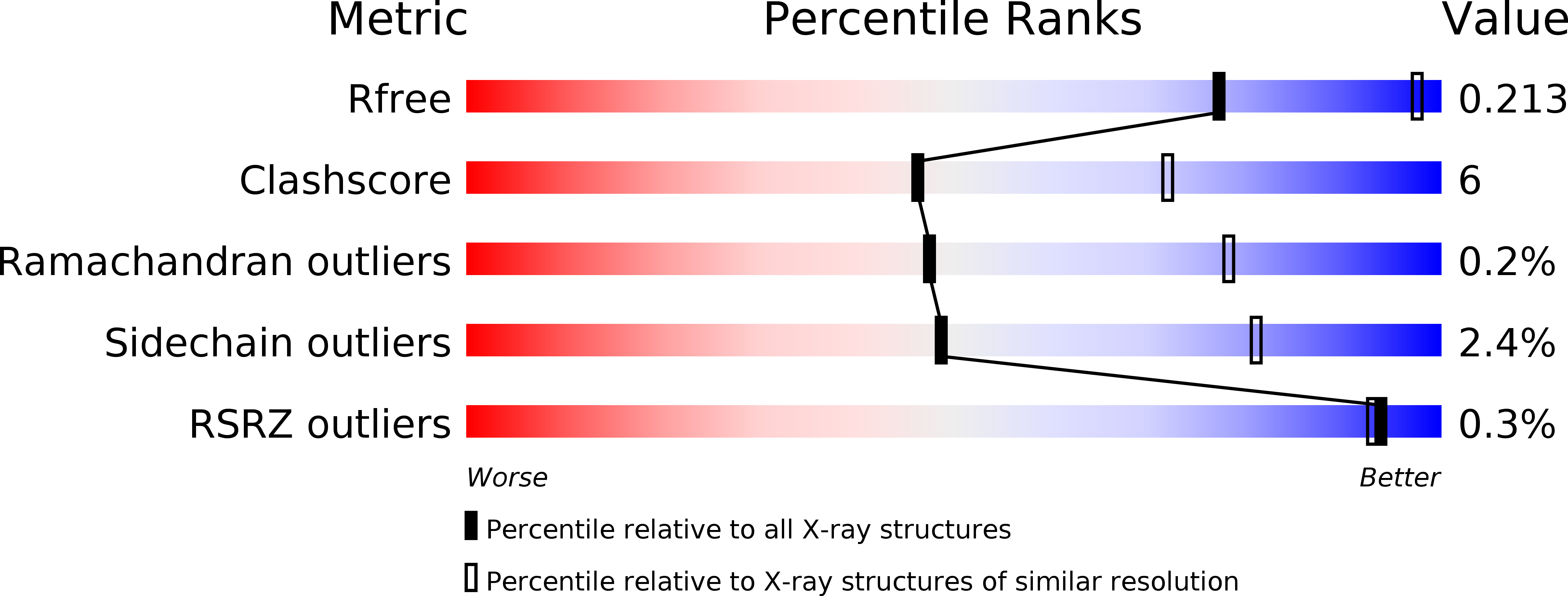
Physical Mechanisms Governing Substituent Effects on Arene-Arene Interactions in a Protein Milieu.
Andersson, C.D., Mishra, B.K., Forsgren, N., Ekstrom, F., Linusson, A.(2020) J Phys Chem B 124: 6529-6539
- PubMed: 32610016
- DOI: https://doi.org/10.1021/acs.jpcb.0c03778
- Primary Citation of Related Structures:
6TD2 - PubMed Abstract:
Arene-arene interactions play important roles in protein-ligand complex formation. Here, we investigate the characteristics of arene-arene interactions between small organic molecules and aromatic amino acids in protein interiors. The study is based on X-ray crystallographic data and quantum mechanical calculations using the enzyme acetylcholinesterase and selected inhibitory ligands as a model system. It is shown that the arene substituents of the inhibitors dictate the strength of the interaction and the geometry of the resulting complexes. Importantly, the calculated interaction energies correlate well with the measured inhibitor potency. Non-hydrogen substituents strengthened all interaction types in the protein milieu, in keeping with results for benzene dimer model systems. The interaction energies were dispersion-dominated, but substituents that induced local dipole moments increased the electrostatic contribution and thus yielded more strongly bound complexes. These findings provide fundamental insights into the physical mechanisms governing arene-arene interactions in the protein milieu and thus into molecular recognition between proteins and small molecules.
Organizational Affiliation:
Department of Chemistry, Umeå University, SE-90187 Umeå, Sweden.