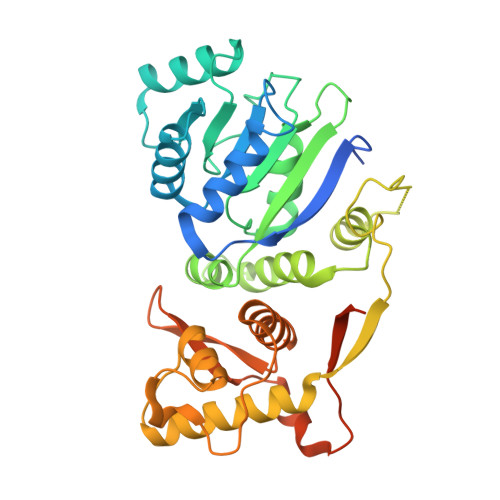
Residues at the interface between zinc binding and winged helix domains of human RECQ1 play a significant role in DNA strand annealing activity.
Mukhopadhyay, S., Das, T., Bose, M., Jain, C.K., Chakraborty, M., Mukherjee, S., Shikha, K., Das, A.K., Ganguly, A.(2021) Nucleic Acids Res
- PubMed: 34751402
- DOI: https://doi.org/10.1093/nar/gkab968
- Primary Citation of Related Structures:
6JTZ - PubMed Abstract:
RECQ1 is the shortest among the five human RecQ helicases comprising of two RecA like domains, a zinc-binding domain and a RecQ C-terminal domain containing the winged-helix (WH). Mutations or deletions on the tip of a β-hairpin located in the WH domain are known to abolish the unwinding activity. Interestingly, the same mutations on the β-hairpin of annealing incompetent RECQ1 mutant (RECQ1T1) have been reported to restore its annealing activity. In an attempt to unravel the strand annealing mechanism, we have crystallized a fragment of RECQ1 encompassing D2-Zn-WH domains harbouring mutations on the β-hairpin. From our crystal structure data and interface analysis, we have demonstrated that an α-helix located in zinc-binding domain potentially interacts with residues of WH domain, which plays a significant role in strand annealing activity. We have shown that deletion of the α-helix or mutation of specific residues on it restores strand annealing activity of annealing deficient constructs of RECQ1. Our results also demonstrate that mutations on the α-helix induce conformational changes and affects DNA stimulated ATP hydrolysis and unwinding activity of RECQ1. Our study, for the first time, provides insight into the conformational requirements of the WH domain for efficient strand annealing by human RECQ1.
Organizational Affiliation:
Department of Biotechnology, Indian Institute of Technology Kharagpur, India.