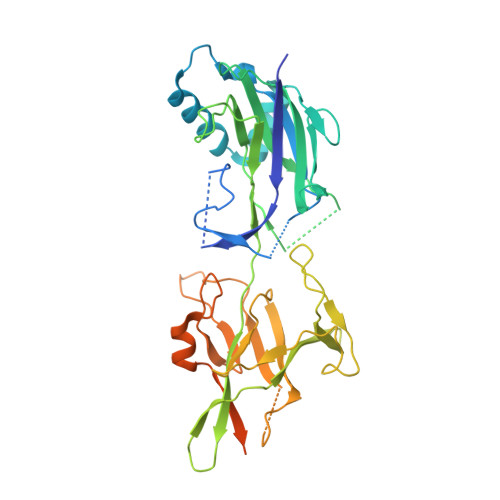
Crystal structure of basal pilin SpaE reveals the molecular basis of its incorporation in the lactobacillar SpaFED pilus.
Megta, A.K., Mishra, A.K., Palva, A., von Ossowski, I., Krishnan, V.(2019) J Struct Biol 207: 74-84
- PubMed: 31026587
- DOI: https://doi.org/10.1016/j.jsb.2019.04.016
- Primary Citation of Related Structures:
6JBV, 6JCH, 6JK7 - PubMed Abstract:
For some Gram-positive genera and species, the long-extended and adhesive sortase-dependent pilus plays an essential role during host colonization, biofilm formation, and immune modulation. Lactobacillus rhamnosus GG is a gut-adapted commensal strain that harbors the operonic genes for the SpaCBA and SpaFED pili, both being comprised of three different protein subunits termed the backbone, tip, and basal pilins. Crystal structures of the backbone pilins (SpaA and SpaD) have recently been solved, and here we describe the high-resolution (1.5 Å) structural determination of the SpaE basal pilin. SpaE consists of two immunoglobulin-like CnaB domains, with each displaying a spontaneously formed internal isopeptide bond, though apparently slow forming in the N-terminal domain. Remarkably, SpaE contains an atypically lengthy unstructured C-terminal tail, along with an YPKN pilin motif peptide, which is normally reserved for backbone subunits. Based on our analysis of the crystal structure data, we provide a molecular model for the basal positioning of the SpaE pilin within the SpaFED pilus.
Organizational Affiliation:
Laboratory of Structural Biology, Regional Centre for Biotechnology, NCR Biotech Science Cluster, Faridabad, Haryana 121 001, India; School of Biotechnology, KIIT University, Odisha 751024, India.