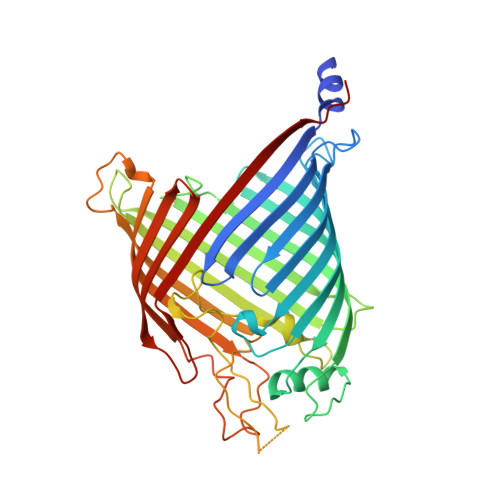
Sequence-Specific Solution NMR Assignments of the beta-Barrel Insertase BamA to Monitor Its Conformational Ensemble at the Atomic Level.
Hartmann, J.B., Zahn, M., Burmann, I.M., Bibow, S., Hiller, S.(2018) J Am Chem Soc 140: 11252-11260
- PubMed: 30125090
- DOI: https://doi.org/10.1021/jacs.8b03220
- Primary Citation of Related Structures:
6FSU - PubMed Abstract:
β-barrel outer membrane proteins (Omps) are key functional components of the outer membranes of Gram-negative bacteria, mitochondria, and plastids. In bacteria, their biogenesis requires the β-barrel-assembly machinery (Bam) with the central insertase BamA, but the exact translocation and insertion mechanism remains elusive. The BamA insertase features a loosely closed gating region between the first and last β-strand 16. Here, we describe ∼70% complete sequence-specific NMR resonance assignments of the transmembrane region of the BamA β-barrel in detergent micelles. On the basis of the assignments, NMR spectra show that the BamA barrel populates a conformational ensemble in slow exchange equilibrium, both in detergent micelles and lipid bilayer nanodiscs. Individual conformers can be selected from the ensemble by the introduction of a C-terminal strand extension, single-point mutations, or specific disulfide cross-linkings, and these modifications at the barrel seam are found to be allosterically coupled to sites at the entire barrel circumference. The resonance assignment provides a platform for mechanistic studies of BamA at atomic resolution, as well as for investigating interactions with potential antibiotic drugs and partner proteins.
Organizational Affiliation:
Biozentrum , University of Basel , Klingelbergstrasse 70 , 4056 Basel , Switzerland.