Crystal Structure of Exotoxin A from Aeromonas Pathogenic Species.
Masuyer, G.(2020) Toxins (Basel) 12
- PubMed: 32549399
- DOI: https://doi.org/10.3390/toxins12060397
- Primary Citation of Related Structures:
6Z5H - PubMed Abstract:
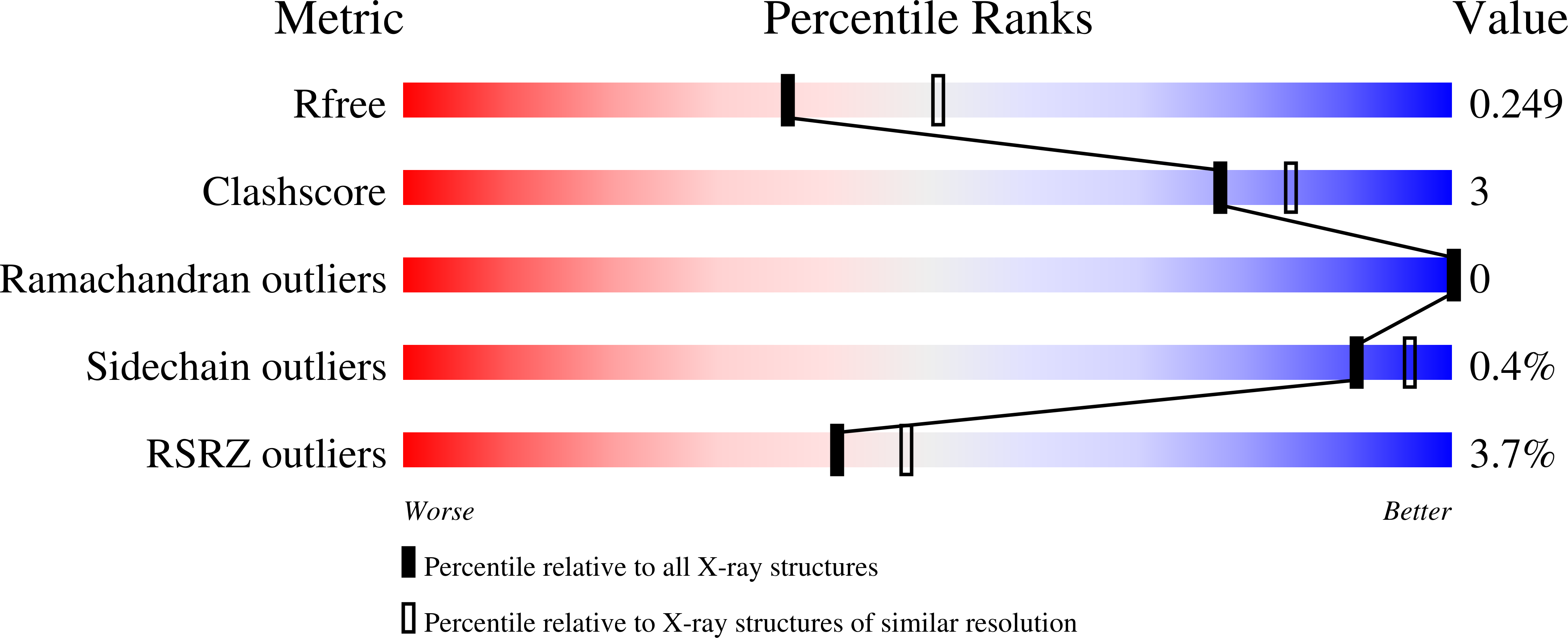
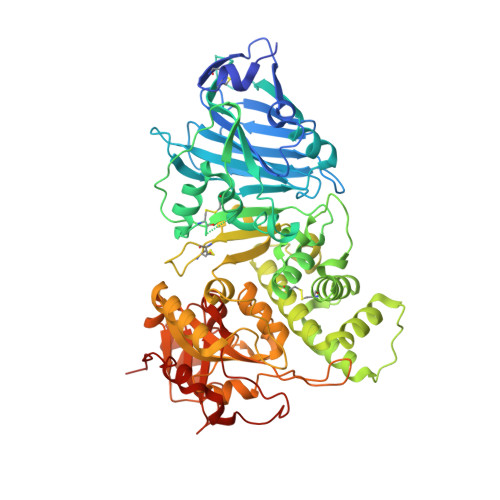
Aeromonas exotoxin A (AE) is a bacterial virulence factor recently discovered in a clinical case of necrotising fasciitis caused by the flesh-eating Aeromonas hydrophila . Here, database mining shows that AE is present in the genome of several emerging Aeromonas pathogenic species. The X-ray crystal structure of AE was solved at 2.3 Å and presents all the hallmarks common to diphthamide-specific mono-ADP-ribosylating toxins, suggesting AE is a fourth member of this family alongside the diphtheria toxin, Pseudomonas exotoxin A and cholix. Structural homology indicates AE may use a similar mechanism of cytotoxicity that targets eukaryotic elongation factor 2 and thus inhibition of protein synthesis. The structure of AE also highlights unique features including a metal binding site, and a negatively charged cleft that could play a role in interdomain interactions and may affect toxicity. This study raises new opportunities to engineer alternative toxin-based molecules with pharmaceutical potential.
Organizational Affiliation:
Department of Pharmacy and Pharmacology, Centre for Therapeutic Innovation, University of Bath, Bath BA2 7AY, UK.