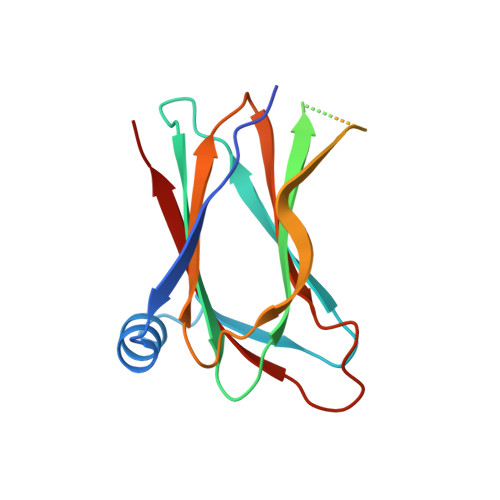
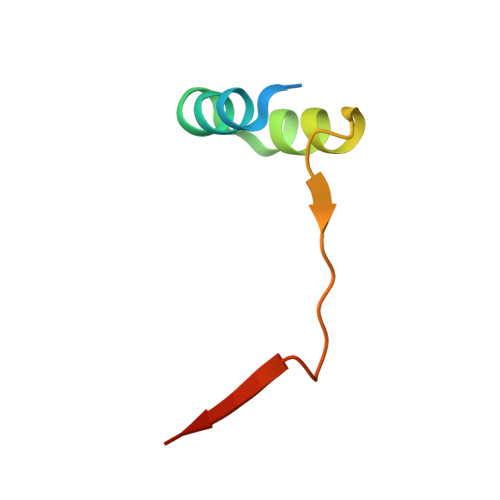
Structural basis for hijacking of the host ACBD3 protein by bovine and porcine enteroviruses and kobuviruses.
Smola, M., Horova, V., Boura, E., Klima, M.(2020) Arch Virol 165: 355-366
- PubMed: 31845156
- DOI: https://doi.org/10.1007/s00705-019-04490-9
- Primary Citation of Related Structures:
6Q67, 6Q68, 6Q69 - PubMed Abstract:
Picornaviruses infect a wide range of mammals including livestock such as cattle and swine. As with other picornavirus genera such as Aphthovirus, there is emerging evidence of a significant economic impact of livestock infections caused by members of the genera Enterovirus and Kobuvirus. While the human-infecting enteroviruses and kobuviruses have been intensively studied during the past decades in great detail, research on livestock-infecting viruses has been mostly limited to the genomic characterization of the viral strains identified worldwide. Here, we extend our previous studies of the structure and function of the complexes composed of the non-structural 3A proteins of human-infecting enteroviruses and kobuviruses and the host ACBD3 protein and present a structural and functional characterization of the complexes of the following livestock-infecting picornaviruses: bovine enteroviruses EV-E and EV-F, porcine enterovirus EV-G, and porcine kobuvirus AiV-C. We present a series of crystal structures of these complexes and demonstrate the role of these complexes in facilitation of viral replication.
Organizational Affiliation:
Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Prague, Czech Republic.