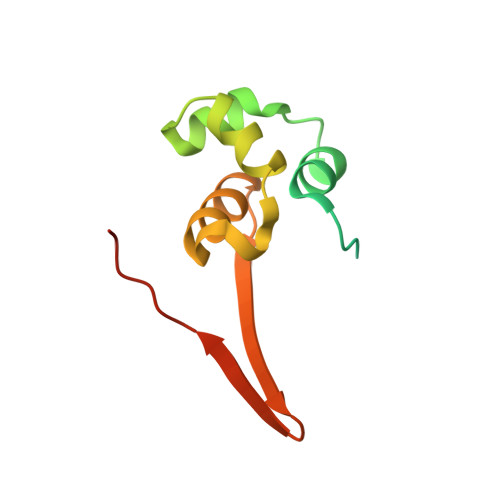
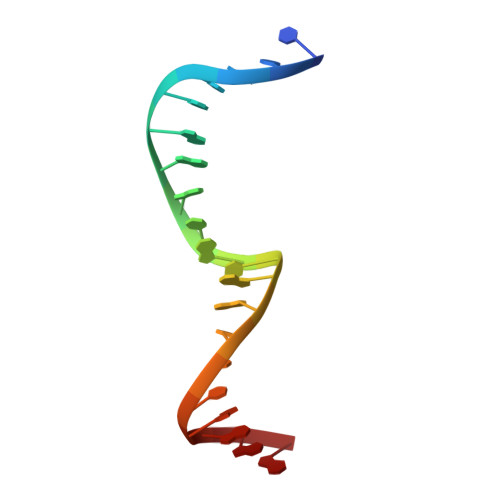
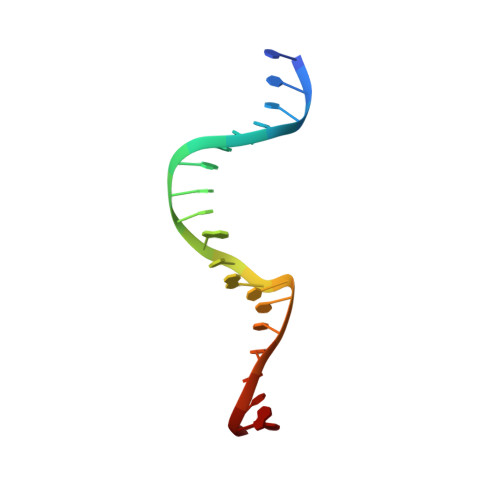
Induced DNA bending by unique dimerization of HigA antitoxin.
Park, J.Y., Kim, H.J., Pathak, C., Yoon, H.J., Kim, D.H., Park, S.J., Lee, B.J.(2020) IUCrJ 7: 748-760
- PubMed: 32695421
- DOI: https://doi.org/10.1107/S2052252520006466
- Primary Citation of Related Structures:
6LTY, 6LTZ - PubMed Abstract:
The bacterial toxin-antitoxin (TA) system regulates cell growth under various environmental stresses. Mycobacterium tuberculosis , the causative pathogen of tuberculosis (TB), has three HigBA type II TA systems with reverse gene organization, consisting of the toxin protein HigB and labile antitoxin protein HigA. Most type II TA modules are transcriptionally autoregulated by the antitoxin itself. In this report, we first present the crystal structure of the M. tuberculosis HigA3 antitoxin ( Mt HigA3) and Mt HigA3 bound to its operator DNA complex. We also investigated the interaction between Mt HigA3 and DNA using NMR spectroscopy. The Mt HigA3 antitoxin structure is a homodimer that contains a structurally well conserved DNA-binding domain at the N-terminus and a dimerization domain at the C-terminus. Upon comparing the HigA homologue structures, a distinct difference was found in the C-terminal region that possesses the β-lid, and diverse orientations of two helix-turn-helix (HTH) motifs from HigA homologue dimers were observed. The structure of Mt HigA3 bound to DNA reveals that the promoter DNA is bound to two HTH motifs of the Mt HigA3 dimer presenting 46.5° bending, and the distance between the two HTH motifs of each Mt HigA3 monomer was increased in Mt HigA3 bound to DNA. The β-lid, which is found only in the tertiary structure of Mt HigA3 among the HigA homologues, causes the formation of a tight dimerization network and leads to a unique arrangement for dimer formation that is related to the curvature of the bound DNA. This work could contribute to the understanding of the HigBA system of M. tuberculosis at the atomic level and may contribute to the development of new antibiotics for TB treatment.
Organizational Affiliation:
Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea.