Diverse Mechanisms of CRISPR-Cas9 Inhibition by Type IIC Anti-CRISPR Proteins.
Zhu, Y., Gao, A., Zhan, Q., Wang, Y., Feng, H., Liu, S., Gao, G., Serganov, A., Gao, P.(2019) Mol Cell 74: 296-309.e7
- PubMed: 30850331
- DOI: https://doi.org/10.1016/j.molcel.2019.01.038
- Primary Citation of Related Structures:
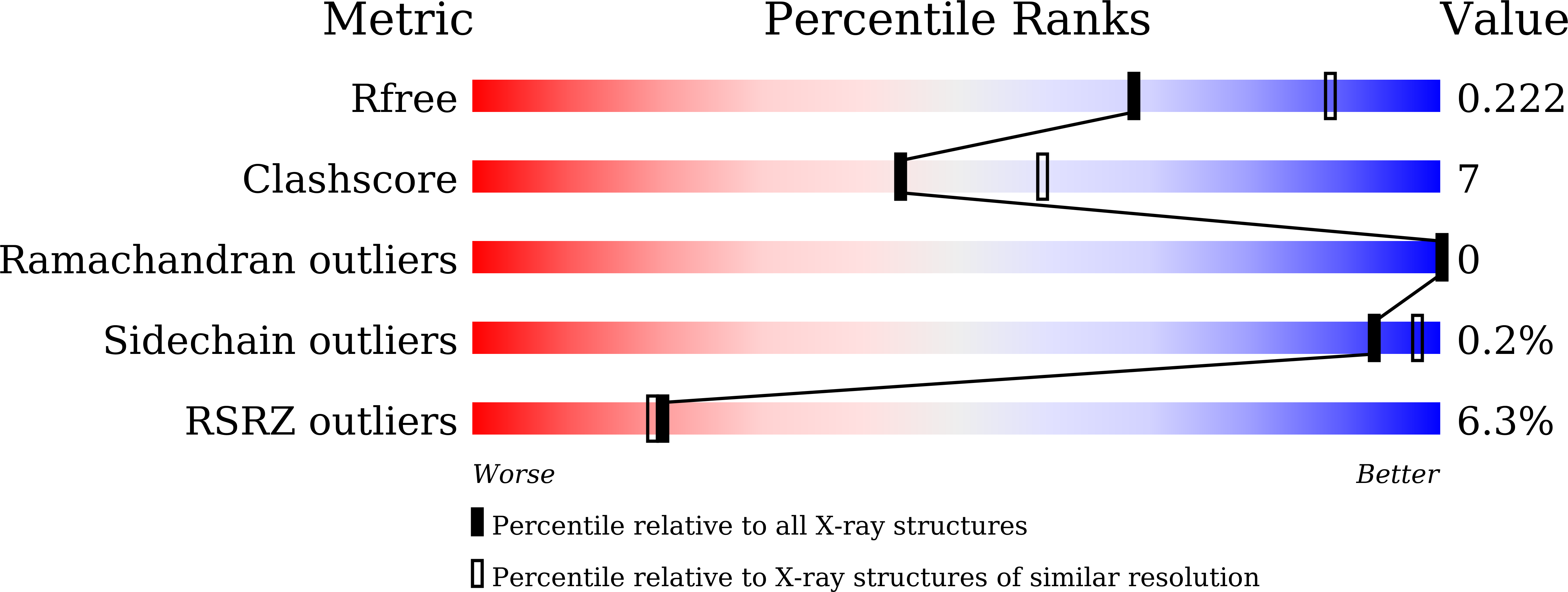
6J9K, 6J9L, 6J9M, 6J9N - PubMed Abstract:
Anti-CRISPR proteins (Acrs) targeting CRISPR-Cas9 systems represent natural "off switches" for Cas9-based applications. Recently, AcrIIC1, AcrIIC2, and AcrIIC3 proteins were found to inhibit Neisseria meningitidis Cas9 (NmeCas9) activity in bacterial and human cells. Here we report biochemical and structural data that suggest molecular mechanisms of AcrIIC2- and AcrIIC3-mediated Cas9 inhibition. AcrIIC2 dimer interacts with the bridge helix of Cas9, interferes with RNA binding, and prevents DNA loading into Cas9. AcrIIC3 blocks the DNA loading step through binding to a non-conserved surface of the HNH domain of Cas9. AcrIIC3 also forms additional interactions with the REC lobe of Cas9 and induces the dimerization of the AcrIIC3-Cas9 complex. While AcrIIC2 targets Cas9 orthologs from different subtypes, albeit with different efficiency, AcrIIC3 specifically inhibits NmeCas9. Structure-guided changes in NmeCas9 orthologs convert them into anti-CRISPR-sensitive proteins. Our studies provide insights into anti-CRISPR-mediated suppression mechanisms and guidelines for designing regulatory tools in Cas9-based applications.
Organizational Affiliation:
CAS Key Laboratory of Infection and Immunity, CAS Center for Excellence in Biomacromolecules, National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China; University of Chinese Academy of Sciences, Beijing 100049, China.