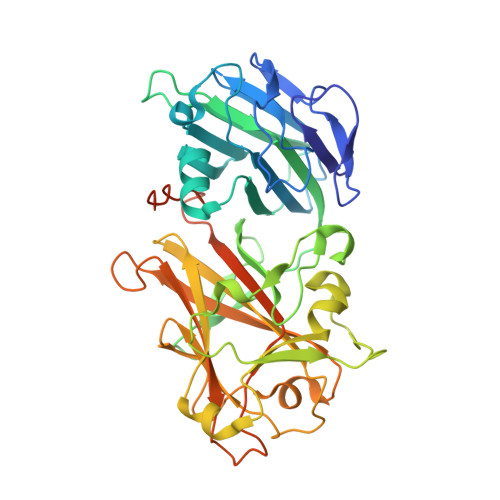
Crystal structures of two tandem malectin-like receptor kinases involved in plant reproduction.
Moussu, S., Augustin, S., Roman, A.O., Broyart, C., Santiago, J.(2018) Acta Crystallogr D Struct Biol 74: 671-680
- PubMed: 29968676
- DOI: https://doi.org/10.1107/S205979831800774X
- Primary Citation of Related Structures:
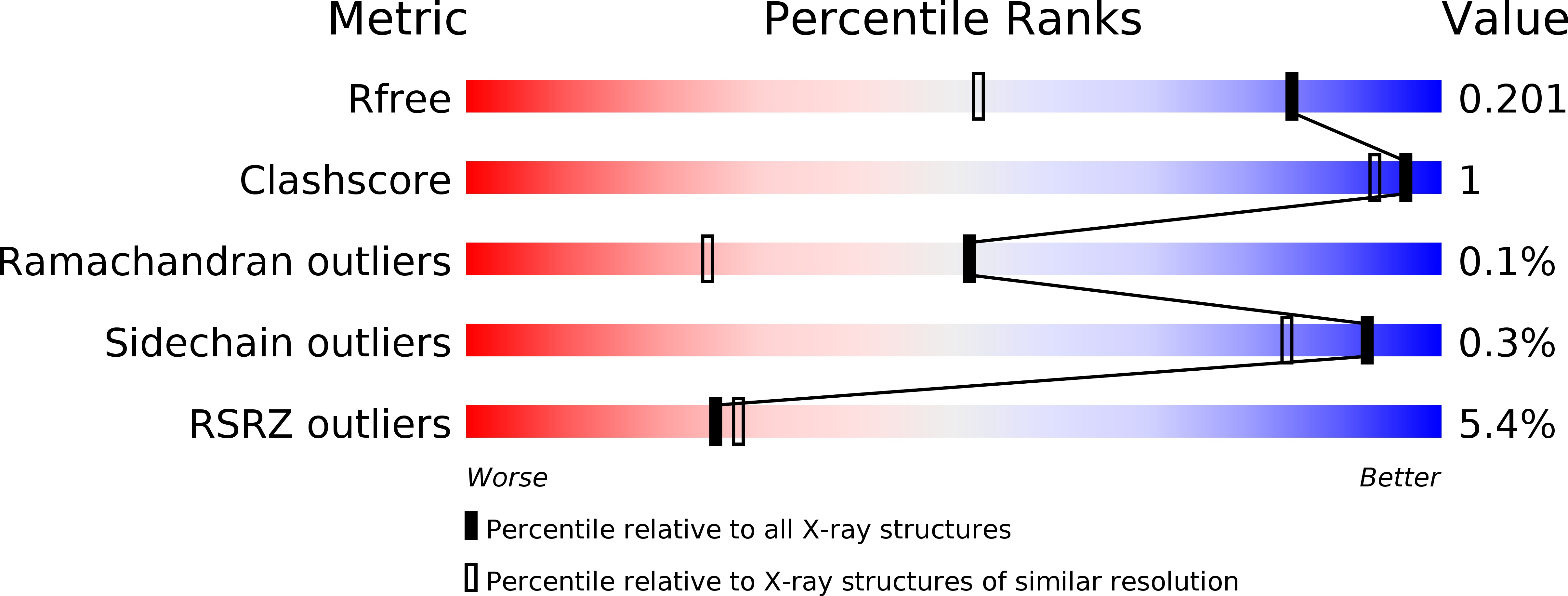
6FIG, 6FIH - PubMed Abstract:
Complex cell-to-cell communication between the male pollen tube and the female reproductive organs is required for plant fertilization. A family of Catharanthus roseus receptor kinase 1-like (CrRLK1L) membrane receptors has been genetically implicated in this process. Here, crystal structures of the CrRLK1Ls ANXUR1 and ANXUR2 are reported at 1.48 and 1.1 Å resolution, respectively. The structures reveal a novel arrangement of two malectin-like domains connected by a short β-hairpin linker and stabilized by calcium ions. The canonical carbohydrate-interaction surfaces of related animal and bacterial carbohydrate-binding modules are not conserved in plant CrRLK1Ls. In line with this, the binding of chemically diverse oligosaccharides to ANXUR1 and HERCULES1 could not be detected. Instead, CrRLK1Ls have evolved a protein-protein interface between their malectin domains which forms a deep cleft lined by highly conserved aromatic and polar residues. Analysis of the glycosylation patterns of different CrRLK1Ls and their oligomeric states suggests that this cleft could resemble a binding site for a ligand required for receptor activation of CrRLK1Ls.
Organizational Affiliation:
The Plant Signaling Mechanisms Laboratory, Department of Plant Molecular Biology, University of Lausanne, 1015 Lausanne, Switzerland.