Engineering the "Missing Link" in Biosynthetic (-)-Menthol Production: Bacterial Isopulegone Isomerase.
Currin, A., Dunstan, M.S., Johannissen, L.O., Hollywood, K.A., Vinaixa, M., Jervis, A.J., Swainston, N., Rattray, N.J.W., Gardiner, J.M., Kell, D.B., Takano, E., Toogood, H.S., Scrutton, N.S.(2018) ACS Catal 8: 2012-2020
- PubMed: 29750129
- DOI: https://doi.org/10.1021/acscatal.7b04115
- Primary Citation of Related Structures:
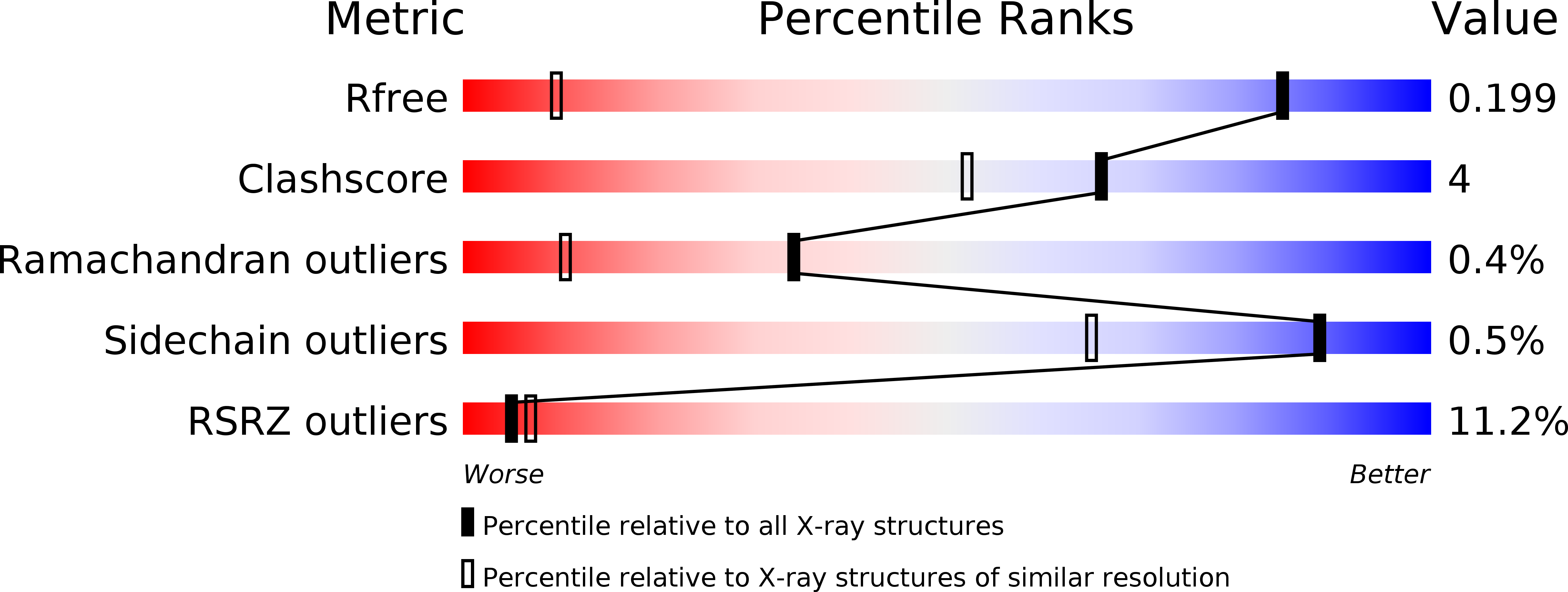
6F4Y, 6F50, 6F53, 6F54 - PubMed Abstract:
The realization of a synthetic biology approach to microbial (1 R ,2 S ,5 R )-( - )-menthol ( 1 ) production relies on the identification of a gene encoding an isopulegone isomerase (IPGI), the only enzyme in the Mentha piperita biosynthetic pathway as yet unidentified. We demonstrate that Δ5-3-ketosteroid isomerase (KSI) from Pseudomonas putida can act as an IPGI, producing ( R )-(+)-pulegone (( R )- 2 ) from (+)- cis -isopulegone ( 3 ). Using a robotics-driven semirational design strategy, we identified a key KSI variant encoding four active site mutations, which confer a 4.3-fold increase in activity over the wild-type enzyme. This was assisted by the generation of crystal structures of four KSI variants, combined with molecular modeling of 3 binding to identify key active site residue targets. The KSI variant was demonstrated to function efficiently within cascade biocatalytic reactions with downstream Mentha enzymes pulegone reductase and (-)-menthone:(-)-menthol reductase to generate 1 from 3 . This study introduces the use of a recombinant IPGI, engineered to function efficiently within a biosynthetic pathway for the production of 1 in microorganisms.
Organizational Affiliation:
Manchester Centre for Fine and Speciality Chemicals (SYNBIOCHEM) and School of Chemistry, Manchester Institute of Biotechnology, University of Manchester, Manchester M1 7DN, United Kingdom.