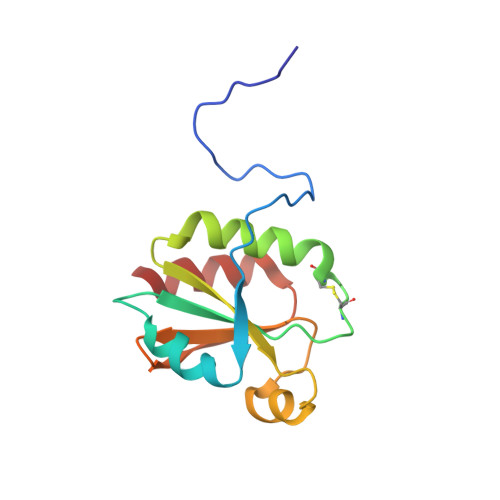
Solution NMR structures of oxidized and reduced Ehrlichia chaffeensis thioredoxin: NMR-invisible structure owing to backbone dynamics.
Buchko, G.W., Hewitt, S.N., Van Voorhis, W.C., Myler, P.J.(2018) Acta Crystallogr F Struct Biol Commun 74: 46-56
- PubMed: 29372907
- DOI: https://doi.org/10.1107/S2053230X1701799X
- Primary Citation of Related Structures:
6ALI, 6AMR - PubMed Abstract:
Thioredoxins are small ubiquitous proteins that participate in a diverse variety of redox reactions via the reversible oxidation of two cysteine thiol groups in a structurally conserved active site. Here, the NMR solution structures of a reduced and oxidized thioredoxin from Ehrlichia chaffeensis (Ec-Trx, ECH_0218), the etiological agent responsible for human monocytic ehrlichiosis, are described. The overall topology of the calculated structures is similar in both redox states and is similar to those of other thioredoxins: a five-stranded, mixed β-sheet (β1-β3-β2-β4-β5) surrounded by four α-helices. Unlike other thioredoxins studied by NMR in both redox states, the 1 H- 15 N HSQC spectrum of reduced Ec-Trx was missing eight additional amide cross peaks relative to the spectrum of oxidized Ec-Trx. These missing amides correspond to residues Cys35-Glu39 in the active-site-containing helix (α2) and Ser72-Ile75 in a loop near the active site, and suggest a change in backbone dynamics on the millisecond-to-microsecond timescale associated with the breakage of an intramolecular Cys32-Cys35 disulfide bond in a thioredoxin. A consequence of the missing amide resonances is the absence of observable or unambiguous NOEs to provide the distance restraints necessary to define the N-terminal end of the α-helix containing the CPGC active site in the reduced state. This region adopts a well defined α-helical structure in other reported reduced thioredoxin structures, is mostly helical in oxidized Ec-Trx and CD studies of Ec-Trx in both redox states suggests there is no significant difference in the secondary structure of the protein. The NMR solution structure of reduced Ec-Trx illustrates that the absence of canonical structure in a region of a protein may be owing to unfavorable dynamics prohibiting NOE observations or unambiguous NOE assignments.
Organizational Affiliation:
Seattle Structural Genomics Center for Infectious Disease, USA.