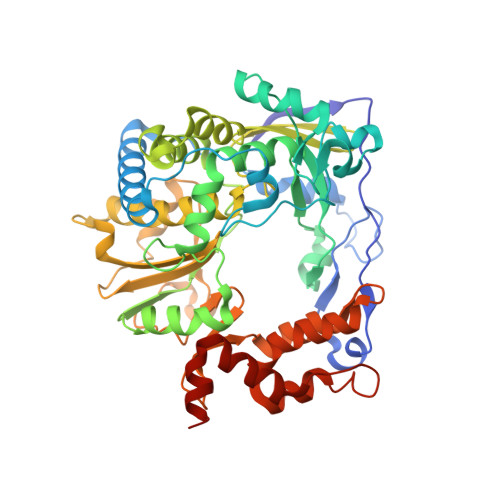
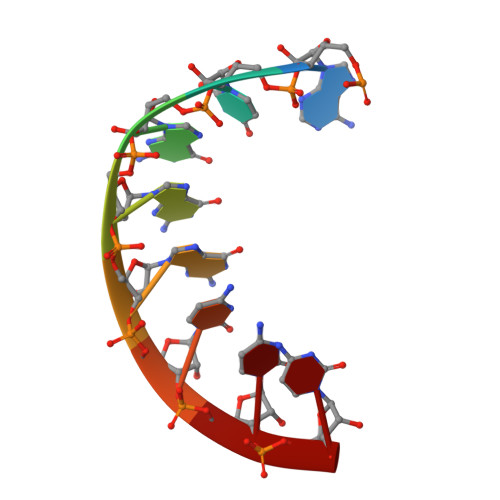
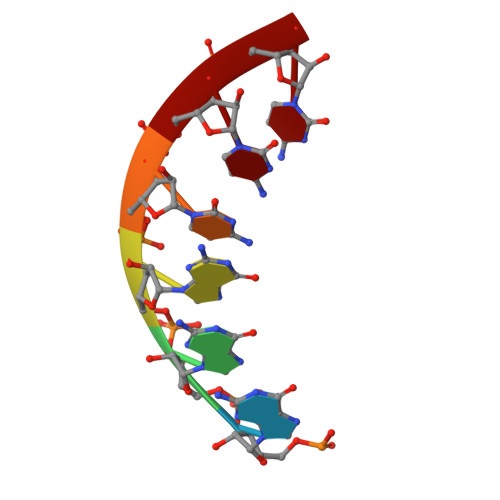
Both cis and trans Activities of Foot-and-Mouth Disease Virus 3D Polymerase Are Essential for Viral RNA Replication.
Herod, M.R., Ferrer-Orta, C., Loundras, E.A., Ward, J.C., Verdaguer, N., Rowlands, D.J., Stonehouse, N.J.(2016) J Virol 90: 6864-6883
- PubMed: 27194768
- DOI: https://doi.org/10.1128/JVI.00469-16
- Primary Citation of Related Structures:
5JXS - PubMed Abstract:
The Picornaviridae is a large family of positive-sense RNA viruses that contains numerous human and animal pathogens, including foot-and-mouth disease virus (FMDV). The picornavirus replication complex comprises a coordinated network of protein-protein and protein-RNA interactions involving multiple viral and host-cellular factors. Many of the proteins within the complex possess multiple roles in viral RNA replication, some of which can be provided in trans (i.e., via expression from a separate RNA molecule), while others are required in cis (i.e., expressed from the template RNA molecule). In vitro studies have suggested that multiple copies of the RNA-dependent RNA polymerase (RdRp) 3D are involved in the viral replication complex. However, it is not clear whether all these molecules are catalytically active or what other function(s) they provide. In this study, we aimed to distinguish between catalytically active 3D molecules and those that build a replication complex. We report a novel nonenzymatic cis-acting function of 3D that is essential for viral-genome replication. Using an FMDV replicon in complementation experiments, our data demonstrate that this cis-acting role of 3D is distinct from the catalytic activity, which is predominantly trans acting. Immunofluorescence studies suggest that both cis- and trans-acting 3D molecules localize to the same cellular compartment. However, our genetic and structural data suggest that 3D interacts in cis with RNA stem-loops that are essential for viral RNA replication. This study identifies a previously undescribed aspect of picornavirus replication complex structure-function and an important methodology for probing such interactions further.
Organizational Affiliation:
School of Molecular and Cellular Biology, Faculty of Biological Sciences and Astbury Centre for Structural Molecular Biology, University of Leeds, Leeds, United Kingdom.