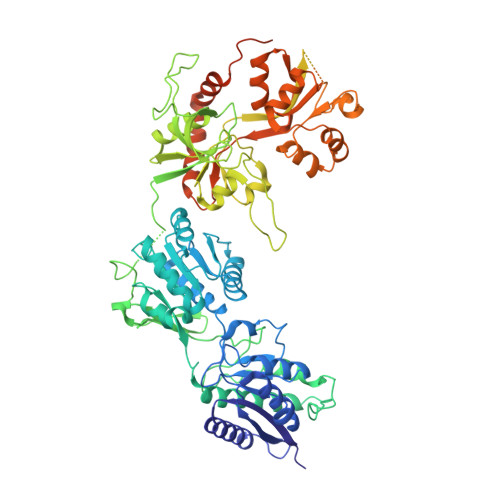
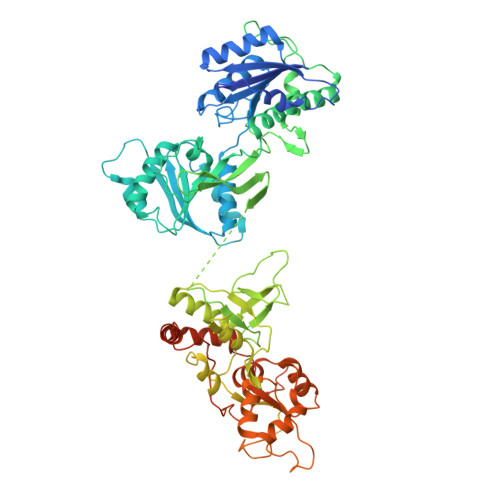
Mechanism of NMDA Receptor Inhibition and Activation.
Zhu, S., Stein, R.A., Yoshioka, C., Lee, C.H., Goehring, A., Mchaourab, H.S., Gouaux, E.(2016) Cell 165: 704-714
- PubMed: 27062927
- DOI: https://doi.org/10.1016/j.cell.2016.03.028
- Primary Citation of Related Structures:
5IOU, 5IOV, 5IPQ, 5IPR, 5IPS, 5IPT, 5IPU, 5IPV - PubMed Abstract:
N-methyl-D-aspartate receptors (NMDARs) are glutamate-gated, calcium-permeable ion channels that mediate synaptic transmission and underpin learning and memory. NMDAR dysfunction is directly implicated in diseases ranging from seizure to ischemia. Despite its fundamental importance, little is known about how the NMDAR transitions between inactive and active states and how small molecules inhibit or activate ion channel gating. Here, we report electron cryo-microscopy structures of the GluN1-GluN2B NMDA receptor in an ensemble of competitive antagonist-bound states, an agonist-bound form, and a state bound with agonists and the allosteric inhibitor Ro25-6981. Together with double electron-electron resonance experiments, we show how competitive antagonists rupture the ligand binding domain (LBD) gating "ring," how agonists retain the ring in a dimer-of-dimers configuration, and how allosteric inhibitors, acting within the amino terminal domain, further stabilize the LBD layer. These studies illuminate how the LBD gating ring is fundamental to signal transduction and gating in NMDARs.
Organizational Affiliation:
Vollum Institute, Oregon Health and Science University, 3181 SW Sam Jackson Park Road, Portland, OR 97239, USA.