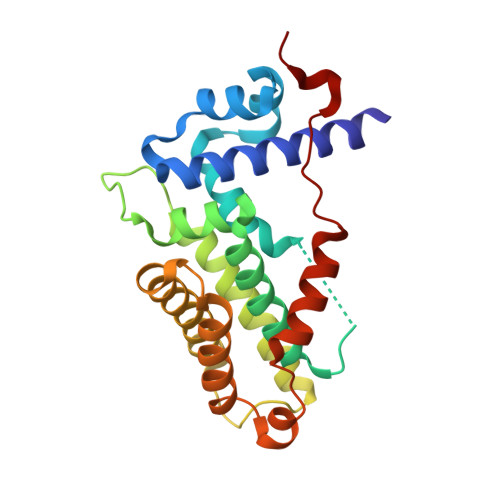
Functional insights into the mode of DNA and ligand binding of the TetR family regulator TylP from Streptomyces fradiae
Ray, S., Maitra, A., Biswas, A., Panjikar, S., Mondal, J., Anand, R.(2017) J Biol Chem 292: 15301-15311
- PubMed: 28739805
- DOI: https://doi.org/10.1074/jbc.M117.788000
- Primary Citation of Related Structures:
5XAY, 5XAZ - PubMed Abstract:
Tetracycline repressors (TetRs) modulate multidrug efflux pathways in several pathogenic bacteria. In Streptomyces , they additionally regulate secondary metabolic pathways like antibiotic production. For instance, in the antibiotic producer Streptomyces fradiae, a layered network of TetRs regulates the levels of the commercially important antibiotic tylosin, with TylP occupying the top of this cascading network. TetRs exist in two functional states, the DNA-bound and the ligand-bound form, which are allosterically regulated. Here, to develop deeper insights into the factors that govern allostery, the crystal structure of TylP was solved to a resolution of 2.3 Å. The structure revealed that TylP possesses several unique features; notably, it harbors a unique C-terminal helix-loop extension that spans the entire length of the structure. This anchor connects the DNA-binding domain (DBD) with the ligand-binding domain (LBD) via a mix of positively charged and hydrogen-bonding interactions. Supporting EMSA studies with a series of ΔC truncated versions show that a systematic deletion of this region results in complete loss of DNA binding. The structure additionally revealed that TylP is markedly different in the orientation of its DBD and LBD architecture and the dimeric geometry from its hypothesized Streptomyces homologue CprB, which is a γ-butyrolactone regulator. Rather, TylP is closer in structural design to macrolide-binding TetRs found in pathogens. Supporting molecular dynamic studies suggested that TylP binds a macrolide intermediate in the tylosin pathway. Collectively, the structure along with corroborating biochemical studies provided insights into the novel mode of regulation of TetRs in antibiotic-producing organisms.
Organizational Affiliation:
From the Department of Chemistry, Indian Institute of Technology Bombay, Mumbai-400076, India.