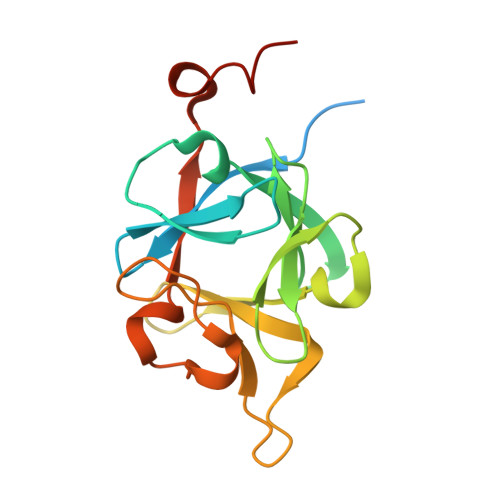
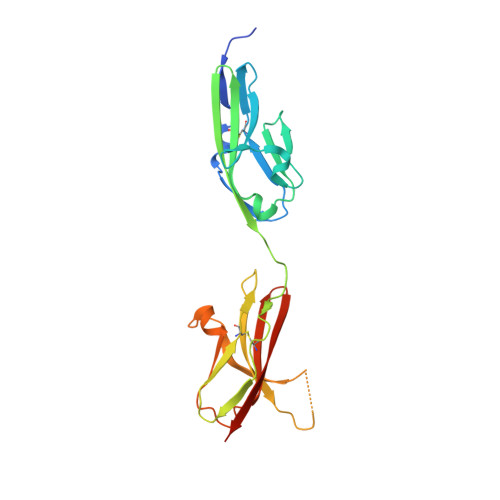Regulation of Receptor Binding Specificity of FGF9 by an Autoinhibitory Homodimerization.
Liu, Y., Ma, J., Beenken, A., Srinivasan, L., Eliseenkova, A.V., Mohammadi, M.(2017) Structure 25: 1325-1336.e3
- PubMed: 28757146
- DOI: https://doi.org/10.1016/j.str.2017.06.016
- Primary Citation of Related Structures:
5W59 - PubMed Abstract:
The epithelial fibroblast growth factor 9 (FGF9) subfamily specifically binds and activates the mesenchymal "c" splice isoform of FGF receptors 1-3 (FGFR1-3) to regulate organogenesis and tissue homeostasis. The unique N and C termini of FGF9 subfamily ligands mediate a reversible homodimerization that occludes major receptor binding sites within the ligand core region. Here we provide compelling X-ray crystallographic, biophysical, and biochemical data showing that homodimerization controls receptor binding specificity of the FGF9 subfamily by keeping the concentration of active FGF9 monomers at a level, which is sufficient for a normal FGFR "c" isoform binding/signaling, but is insufficient for an illegitimate FGFR "b" isoform binding/signaling. We show that deletion of the N terminus or alanine substitutions in the C terminus of FGF9 skews the delicate ligand equilibrium toward active FGF9 monomers causing off-target binding and activation of FGFR b isoforms. Our study is the first to implicate ligand homodimerization in the regulation of ligand-receptor specificity.
Organizational Affiliation:
Department of Biochemistry & Molecular Pharmacology, New York University School of Medicine, New York, NY 10016, USA.