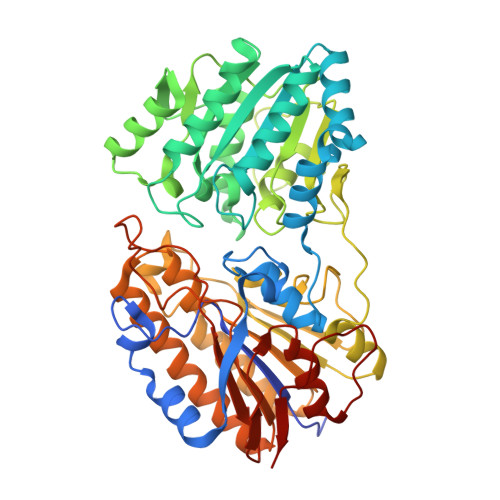
Crystal Structure of 2,3-bisphosphoglycerate-independent phosphoglycerate mutase bound to 3-phosphoglycerate, from Acinetobacter baumannii
Delker, S.L., Dranow, D.M., Abendroth, J., Lorimer, D., Edwards, T.E., Seattle Structural Genomics Center for Infectious Disease (SSGCID)To be published.