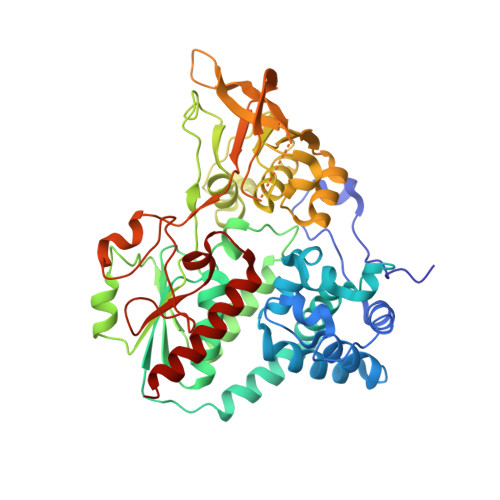
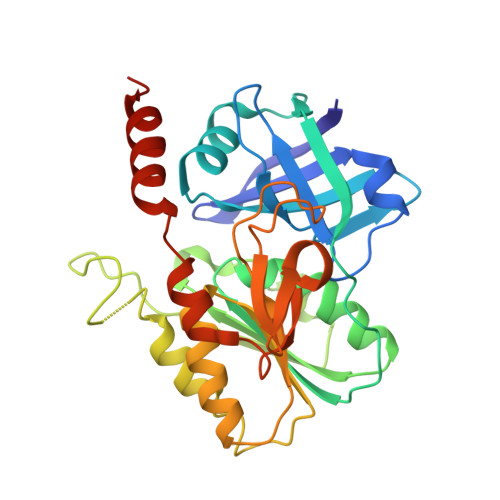
Two functionally distinct NADP(+)-dependent ferredoxin oxidoreductases maintain the primary redox balance of Pyrococcus furiosus.
Nguyen, D.M.N., Schut, G.J., Zadvornyy, O.A., Tokmina-Lukaszewska, M., Poudel, S., Lipscomb, G.L., Adams, L.A., Dinsmore, J.T., Nixon, W.J., Boyd, E.S., Bothner, B., Peters, J.W., Adams, M.W.W.(2017) J Biol Chem 292: 14603-14616
- PubMed: 28705933
- DOI: https://doi.org/10.1074/jbc.M117.794172
- Primary Citation of Related Structures:
5VJ7 - PubMed Abstract:
Electron bifurcation has recently gained acceptance as the third mechanism of energy conservation in which energy is conserved through the coupling of exergonic and endergonic reactions. A structure-based mechanism of bifurcation has been elucidated recently for the flavin-based enzyme NADH-dependent ferredoxin NADP + oxidoreductase I (NfnI) from the hyperthermophillic archaeon Pyrococcus furiosus. NfnI is thought to be involved in maintaining the cellular redox balance, producing NADPH for biosynthesis by recycling the two other primary redox carriers, NADH and ferredoxin. The P. furiosus genome encodes an NfnI paralog termed NfnII, and the two are differentially expressed, depending on the growth conditions. In this study, we show that deletion of the genes encoding either NfnI or NfnII affects the cellular concentrations of NAD(P)H and particularly NADPH. This results in a moderate to severe growth phenotype in deletion mutants, demonstrating a key role for each enzyme in maintaining redox homeostasis. Despite their similarity in primary sequence and cofactor content, crystallographic, kinetic, and mass spectrometry analyses reveal that there are fundamental structural differences between the two enzymes, and NfnII does not catalyze the NfnI bifurcating reaction. Instead, it exhibits non-bifurcating ferredoxin NADP oxidoreductase-type activity. NfnII is therefore proposed to be a bifunctional enzyme and also to catalyze a bifurcating reaction, although its third substrate, in addition to ferredoxin and NADP(H), is as yet unknown.
Organizational Affiliation:
From the Department of Biochemistry and Molecular Biology, University of Georgia, Athens, Georgia 30602.