Myosin 7 and its adaptors link cadherins to actin.
Yu, I.M., Planelles-Herrero, V.J., Sourigues, Y., Moussaoui, D., Sirkia, H., Kikuti, C., Stroebel, D., Titus, M.A., Houdusse, A.(2017) Nat Commun 8: 15864-15864
- PubMed: 28660889
- DOI: https://doi.org/10.1038/ncomms15864
- Primary Citation of Related Structures:
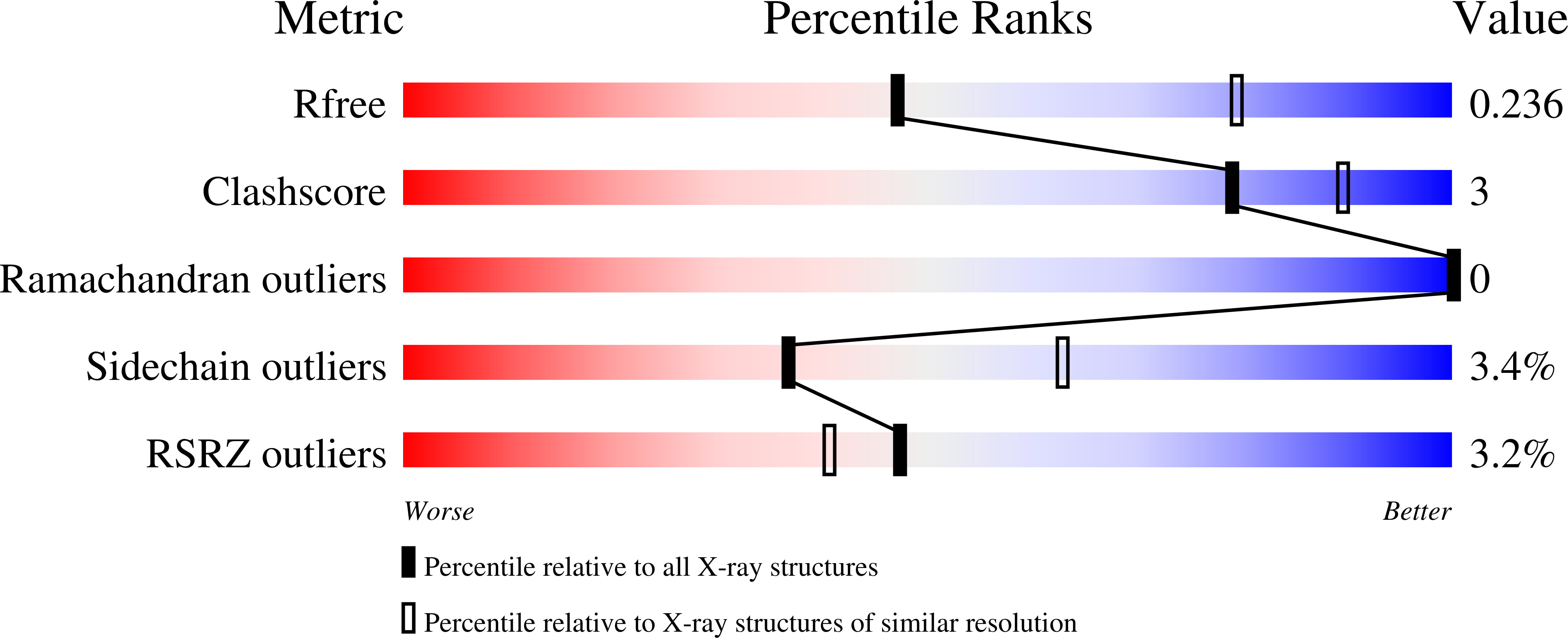
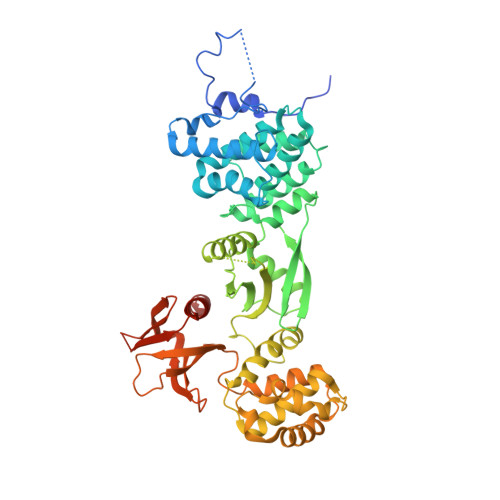
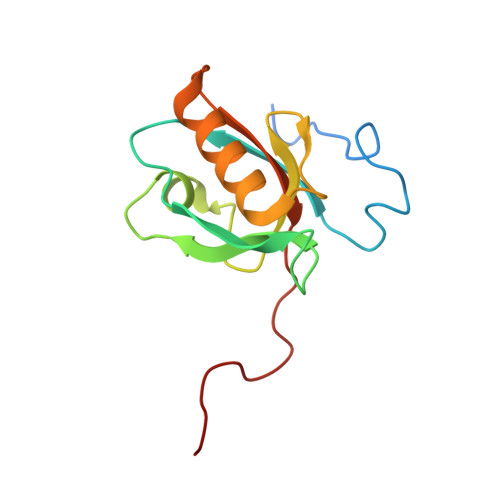
5MV7, 5MV8, 5MV9 - PubMed Abstract:
Cadherin linkages between adjacent stereocilia and microvilli are essential for mechanotransduction and maintaining their organization. They are anchored to actin through interaction of their cytoplasmic domains with related tripartite complexes consisting of a class VII myosin and adaptor proteins: Myo7a/SANS/Harmonin in stereocilia and Myo7b/ANKS4B/Harmonin in microvilli. Here, we determine high-resolution structures of Myo7a and Myo7b C-terminal MyTH4-FERM domain (MF2) and unveil how they recognize harmonin using a novel binding mode. Systematic definition of interactions between domains of the tripartite complex elucidates how the complex assembles and prevents possible self-association of harmonin-a. Several Myo7a deafness mutants that map to the surface of MF2 disrupt harmonin binding, revealing the molecular basis for how they impact the formation of the tripartite complex and disrupt mechanotransduction. Our results also suggest how switching between different harmonin isoforms can regulate the formation of networks with Myo7a motors and coordinate force sensing in stereocilia.
Organizational Affiliation:
Structural Motility, Institut Curie, PSL Research University, CNRS, UMR 144, F-75005 Paris, France.