Thieno[3,2-b]pyrrole-5-carboxamides as New Reversible Inhibitors of Histone Lysine Demethylase KDM1A/LSD1. Part 1: High-Throughput Screening and Preliminary Exploration.
Sartori, L., Mercurio, C., Amigoni, F., Cappa, A., Faga, G., Fattori, R., Legnaghi, E., Ciossani, G., Mattevi, A., Meroni, G., Moretti, L., Cecatiello, V., Pasqualato, S., Romussi, A., Thaler, F., Trifiro, P., Villa, M., Vultaggio, S., Botrugno, O.A., Dessanti, P., Minucci, S., Zagarri, E., Carettoni, D., Iuzzolino, L., Varasi, M., Vianello, P.(2017) J Med Chem 60: 1673-1692
- PubMed: 28186755
- DOI: https://doi.org/10.1021/acs.jmedchem.6b01018
- Primary Citation of Related Structures:
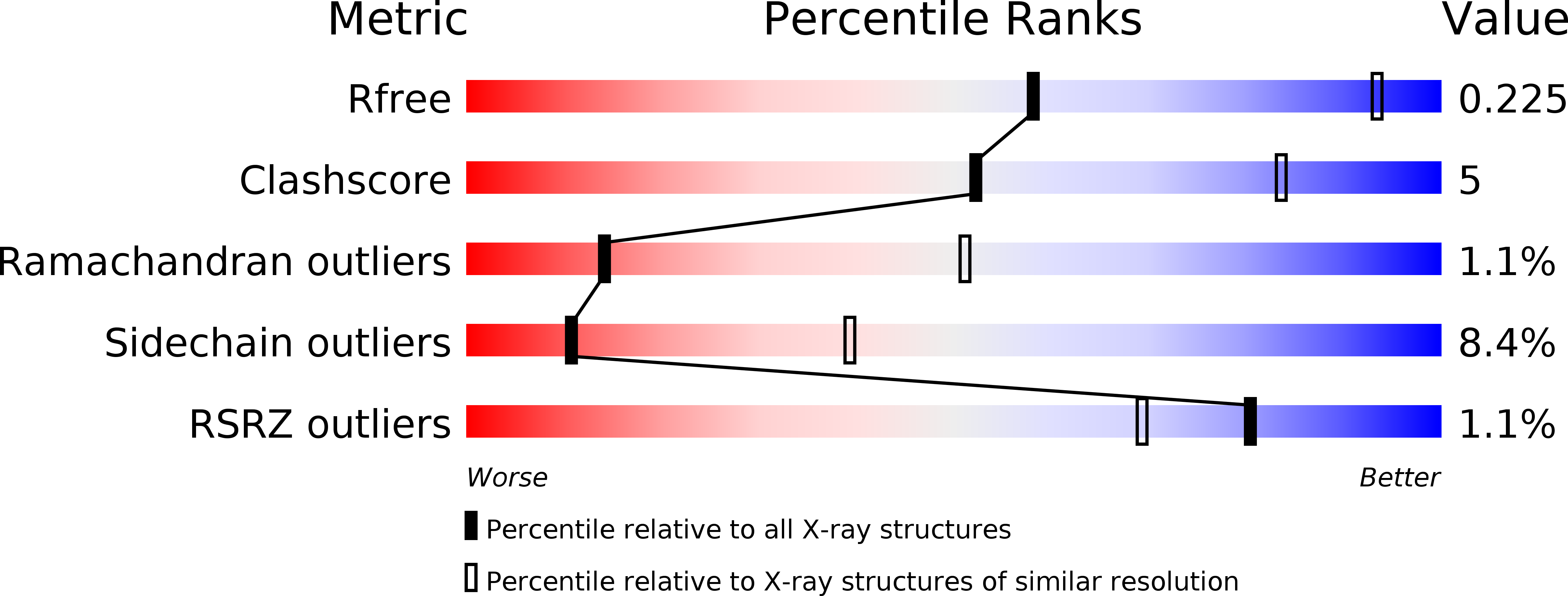
5LGN - PubMed Abstract:
Lysine specific demethylase 1 KDM1A (LSD1) regulates histone methylation and it is increasingly recognized as a potential therapeutic target in oncology. We report on a high-throughput screening campaign performed on KDM1A/CoREST, using a time-resolved fluorescence resonance energy transfer (TR-FRET) technology, to identify reversible inhibitors. The screening led to 115 hits for which we determined biochemical IC 50 , thus identifying four chemical series. After data analysis, we have prioritized the chemical series of N-phenyl-4H-thieno[3, 2-b]pyrrole-5-carboxamide for which we obtained X-ray structures of the most potent hit (compound 19, IC 50 = 2.9 μM) in complex with the enzyme. Initial expansion of this chemical class, both modifying core structure and decorating benzamide moiety, was directed toward the definition of the moieties responsible for the interaction with the enzyme. Preliminary optimization led to compound 90, which inhibited the enzyme with a submicromolar IC 50 (0.162 μM), capable of inhibiting the target in cells.
Organizational Affiliation:
Department of Experimental Oncology, Academic Drug Discovery, European Institute of Oncology , Via Adamello 16, 20139 Milano, Italy.