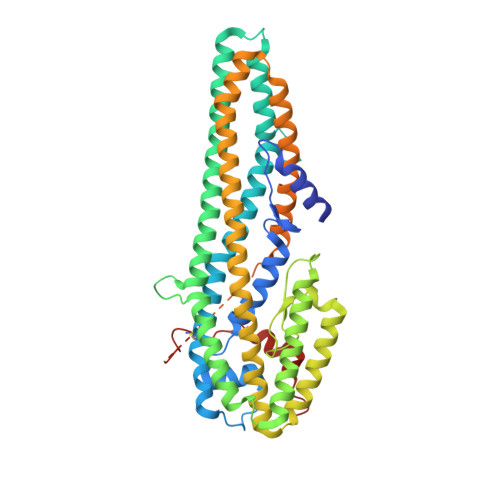
The pesticidal Cry6Aa toxin from Bacillus thuringiensis is structurally similar to HlyE-family alpha pore-forming toxins.
Dementiev, A., Board, J., Sitaram, A., Hey, T., Kelker, M.S., Xu, X., Hu, Y., Vidal-Quist, C., Chikwana, V., Griffin, S., McCaskill, D., Wang, N.X., Hung, S.C., Chan, M.K., Lee, M.M., Hughes, J., Wegener, A., Aroian, R.V., Narva, K.E., Berry, C.(2016) BMC Biol 14: 71-71
- PubMed: 27576487
- DOI: https://doi.org/10.1186/s12915-016-0295-9
- Primary Citation of Related Structures:
5KUC, 5KUD - PubMed Abstract:
The Cry6 family of proteins from Bacillus thuringiensis represents a group of powerful toxins with great potential for use in the control of coleopteran insects and of nematode parasites of importance to agriculture. These proteins are unrelated to other insecticidal toxins at the level of their primary sequences and the structure and function of these proteins has been poorly studied to date. This has inhibited our understanding of these toxins and their mode of action, along with our ability to manipulate the proteins to alter their activity to our advantage. To increase our understanding of their mode of action and to facilitate further development of these proteins we have determined the structure of Cry6Aa in protoxin and trypsin-activated forms and demonstrated a pore-forming mechanism of action.
Organizational Affiliation:
Shamrock Structures LLC, Woodridge, IL, USA.