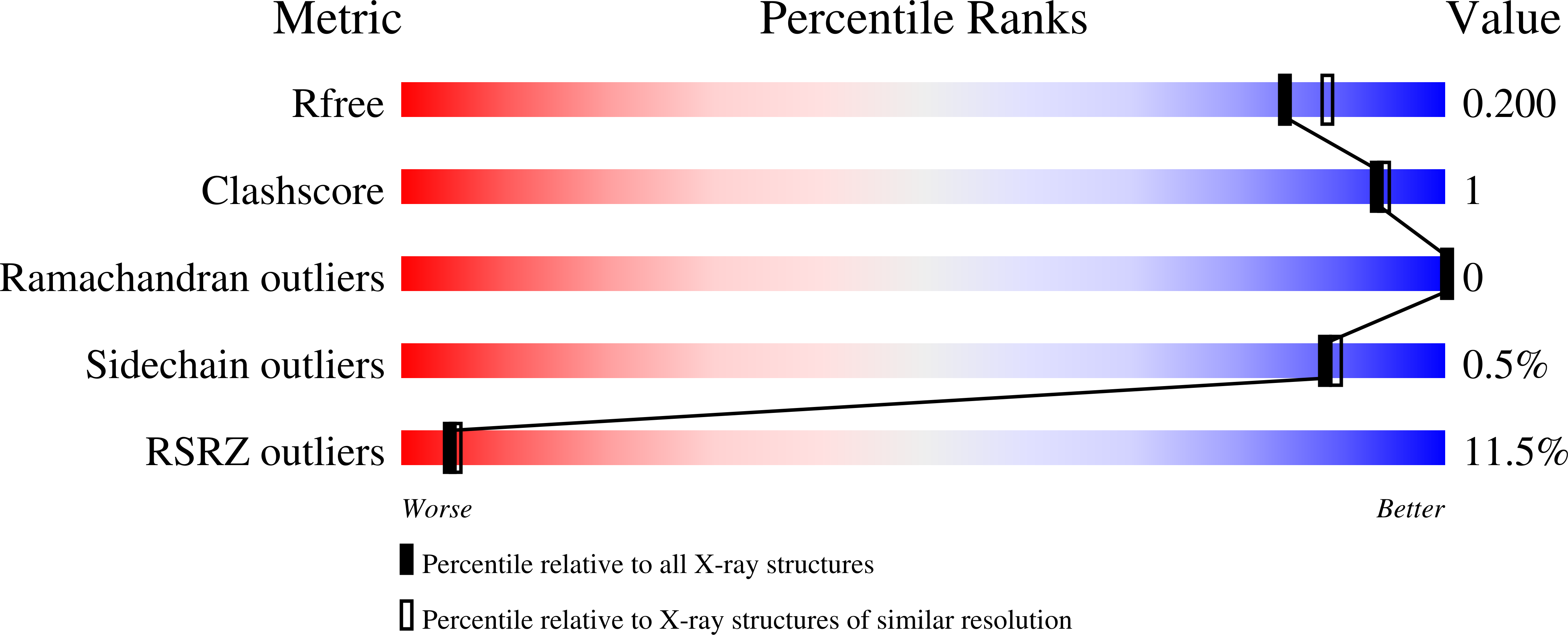
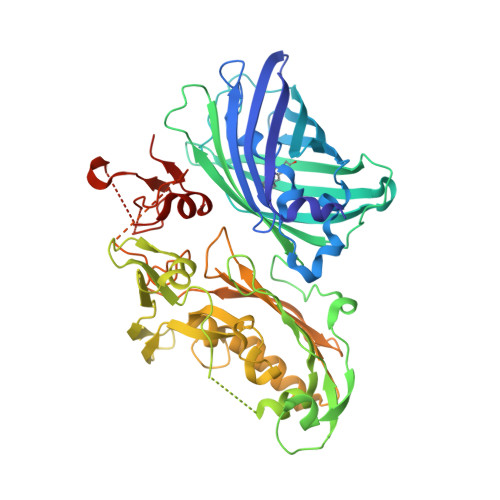
Structural characterization of the virulence factor Sda1 nuclease from Streptococcus pyogenes.
Moon, A.F., Krahn, J.M., Lu, X., Cuneo, M.J., Pedersen, L.C.(2016) Nucleic Acids Res 44: 3946-3957
- PubMed: 26969731
- DOI: https://doi.org/10.1093/nar/gkw143
- Primary Citation of Related Structures:
5FGU, 5FGW - PubMed Abstract:
Infection by Group A Streptococcus pyogenes (GAS) is a leading cause of severe invasive disease in humans, including streptococcal toxic shock syndrome and necrotizing fasciitis. GAS infections lead to nearly 163,000 annual deaths worldwide. Hypervirulent strains of S. pyogenes have evolved a plethora of virulence factors that aid in disease-by promoting bacterial adhesion to host cells, subsequent invasion of deeper tissues and blocking the immune system's attempts to eradicate the infection. Expression and secretion of the extracellular nuclease Sda1 is advantageous for promoting bacterial dissemination throughout the host organism, and evasion of the host's innate immune response. Here we present two crystal structures of Sda1, as well as biochemical studies to address key structural features and surface residues involved in DNA binding and catalysis. In the active site, Asn211 is observed to directly chelate a hydrated divalent metal ion and Arg124, on the putative substrate binding loop, likely stabilizes the transition state during phosphodiester bond cleavage. These structures provide a foundation for rational drug design of small molecule inhibitors to be used in prevention of invasive streptococcal disease.
Organizational Affiliation:
Genome Integrity and Structural Biology Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC 27709, USA moon@niehs.nih.gov.