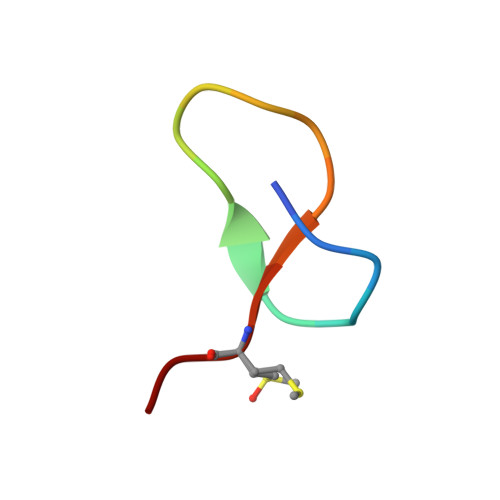
The ring residue proline 8 is crucial for the thermal stability of the lasso peptide caulosegnin II.
Hegemann, J.D., Fage, C.D., Zhu, S., Harms, K., Di Leva, F.S., Novellino, E., Marinelli, L., Marahiel, M.A.(2016) Mol Biosyst 12: 1106-1109
- PubMed: 26863937
- DOI: https://doi.org/10.1039/c6mb00081a
- Primary Citation of Related Structures:
5D9E - PubMed Abstract:
Lasso peptides are fascinating natural products with a unique structural fold that can exhibit tremendous thermal stability. Here, we investigate factors responsible for the thermal stability of caulosegnin II. By employing X-ray crystallography, mutational analysis and molecular dynamics simulations, the ring residue proline 8 was proven to be crucial for thermal stability.
Organizational Affiliation:
Department of Chemistry/Biochemistry, LOEWE Center for Synthetic Microbiology, Philipps-Universität Marburg, Hans-Meerwein-Strasse 4, 35032 Marburg, Germany. marahiel@staff.uni-marburg.de.