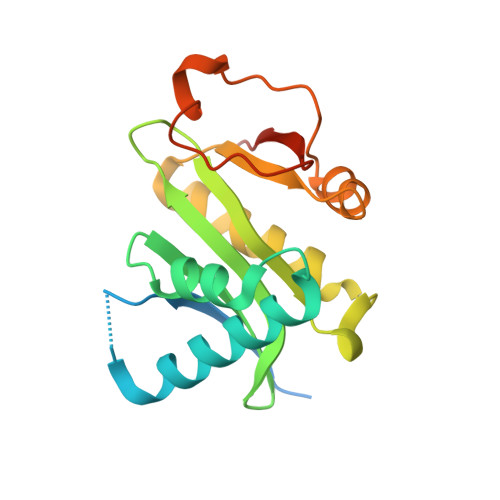
Dimeric structure of p300/CBP associated factor.
Shi, S., Lin, J., Cai, Y., Yu, J., Hong, H., Ji, K., Downey, J.S., Lu, X., Chen, R., Han, J., Han, A.(2014) BMC Struct Biol 14: 2-2
- PubMed: 24423233
- DOI: https://doi.org/10.1186/1472-6807-14-2
- Primary Citation of Related Structures:
4NSQ - PubMed Abstract:
p300/CBP associating factor (PCAF, also known as KAT2B for lysine acetyltransferase 2B) is a catalytic subunit of megadalton metazoan complex ATAC (Ada-Two-A containing complex) for acetylation of histones. However, relatively little is known about the regulation of the enzymatic activity of PCAF.
Organizational Affiliation:
State Key Laboratory of Cellular Stress Biology, School of Life Sciences, Xiamen University, Xiamen 361102, China. jhan@xmu.edu.cn.