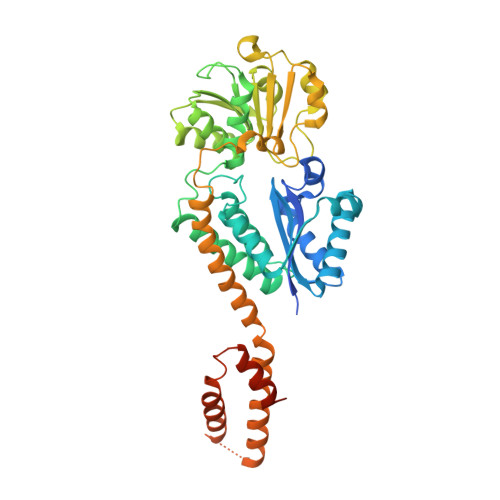
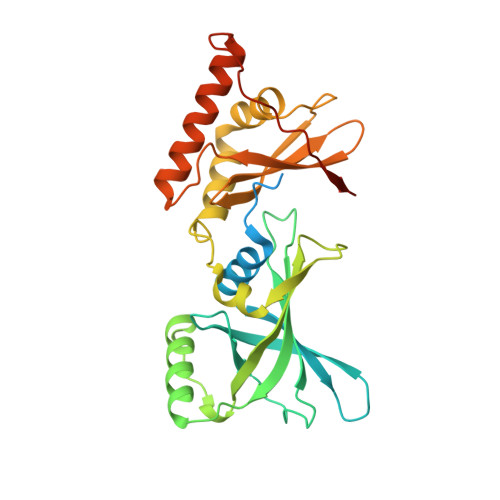
Crystal structure of Arabidopsis glutamyl-tRNA reductase in complex with its stimulator protein
Zhao, A., Fang, Y., Chen, X., Zhao, S., Dong, W., Lin, Y., Gong, W., Liu, L.(2014) Proc Natl Acad Sci U S A 111: 6630-6635
- PubMed: 24753615
- DOI: https://doi.org/10.1073/pnas.1400166111
- Primary Citation of Related Structures:
4N7R - PubMed Abstract:
Tetrapyrrole biosynthesis in plants, algae, and most bacteria starts from the NADPH-dependent reduction of glutamyl-tRNA by glutamyl-tRNA reductase (GluTR). The GluTR-catalyzed reaction is the rate-limiting step, and GluTR is the target of multiple posttranslational regulations, such as heme feedback inhibition, for the tetrapyrrole biosynthetic pathway. A recently identified GluTR regulator, GluTR binding protein (GluBP), has been shown to spatially organize tetrapyrrole synthesis by distributing GluTR into different suborganellar locations. Here we report the complex structure of GluTR-GluBP from Arabidopsis thaliana. The dimeric GluBP binds symmetrically to the catalytic domains of the V-shaped GluTR dimer via its C-terminal domain. A substantial conformational change of the GluTR NADPH-binding domain is observed, confirming the postulated rotation of the NADPH-binding domain for hydride transfer from NADPH to the substrate. Arg146, "guarding the door" for metabolic channeling, adopts alternative conformations, which may represent steps involved in substrate recognition and product release. A coupled enzyme assay shows that GluBP stimulates GluTR catalytic efficiency with an approximate threefold increase of the 5-aminolevulinic acid formation rate. In addition, the GluTR activity can be inhibited by heme in a concentration-dependent way regardless of the presence of GluBP. A structural alignment indicates that GluBP belongs to a heme-binding family involved in heme metabolism. We propose a catalytic mechanism model for GluTR, through which photosynthetic organisms can achieve precise regulation of tetrapyrrole biosynthesis.
Organizational Affiliation:
Photosynthesis Research Center, Key Laboratory of Photobiology, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China.