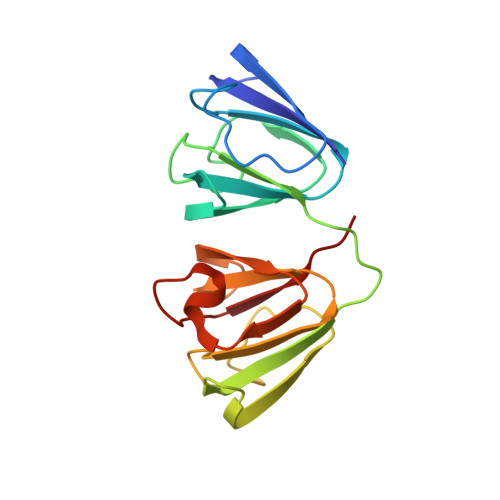
Crystal structure of the cataract-causing P23T gamma D-crystallin mutant.
Ji, F., Koharudin, L.M., Jung, J., Gronenborn, A.M.(2013) Proteins 81: 1493-1498
- PubMed: 23670788
- DOI: https://doi.org/10.1002/prot.24321
- Primary Citation of Related Structures:
4JGF - PubMed Abstract:
Up to now, efforts to crystallize the cataract-associated P23T mutant of human γD-crystallin have not been successful. Therefore, insights into the light scattering mechanism of this mutant have been exclusively obtained from solution work. Here we present the first crystal structure of the P23T mutant at 2.5 Å resolution. The protein exhibits essentially the same overall structure as seen for the wild-type protein. Based on our structural data, we confirm that no major conformational changes are caused by the mutation, and that solution phase properties of the mutant appear exclusively associated with cataract formation.
Organizational Affiliation:
Department of Structural Biology, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, 15261; School of Life Science and Biotechnology, Dalian University of Technology, Lingong Road, Dalian, 16024, China.