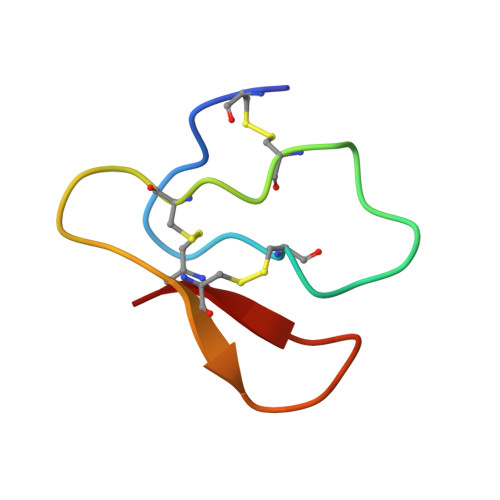
Solution Structure of the Cellulose-Binding Domain of Endoglucanase I from Trichoderma Reesei and its Interaction with Cello-Oligosaccharides.
Mattinen, M.L., Linder, M., Drakenberg, T., Annila, A.(1998) Eur J Biochem 256: 279
- PubMed: 9760165
- DOI: https://doi.org/10.1046/j.1432-1327.1998.2560279.x
- Primary Citation of Related Structures:
4BMF - PubMed Abstract:
The solution structure of a synthetic 38-residue cellulose-binding domain (CBD) of endoglucanase I from Trichoderma reesei (CBD(EGI)) was determined by two-dimensional 1H-NMR spectroscopy. 100 structures were generated from a total of 599 NOE derived distance restraints and 28 phi and 14 chi dihedral angle restraints. For the final set of 19 selected structures, the rms deviation about the mean structure was 0.83+/-0.26 A for all atoms and 0.50+/-0.22 A for the backbone atoms. The structure of CBD(EGI) was very similar to that of CBD of cellobiohydrolase I from T reesei (CBD(CBHI)). The backbone trace of CBD(EGI) followed closely the irregular triple-stranded antiparallel beta-sheet structure of CBD(CBHI). Moreover, apart from the different side chains of Trp7 (CBD(EGI)) and Tyr5 (CBD(CBHI)), the cellulose-binding face of CBD(EGI) was similar to that of CBD(CBHI) within the precision of the structures. Finally, the interaction between CBD(EGI) and soluble sugars was investigated using cellopentaose and cellohexaose as substrates. Experiments showed that the interactions between CBD(EGI) and cellobiose units of sugars are specific, supporting the previously presented model for the CBD binding to crystalline cellulose.
Organizational Affiliation:
VTT Chemical Technology, Finland. Maija.Mattinen@vtt.fi